Chemistry:Nickel hydrazine nitrate
| |
| Names | |
|---|---|
| Other names
Tris(hydrazine)nickel(II) nitrate
Trihydrazinenickel nitrate NHN | |
| Identifiers | |
| Properties | |
| H12N8NiO6 | |
| Molar mass | 278.839 g·mol−1 |
| Appearance | purple solid |
| Boiling point | explode |
| Explosive data | |
| Shock sensitivity | 18.82 J |
| Friction sensitivity | 15.6906 N |
| Detonation velocity | 3,600 m/s @ .8 g/cm3
7,000 m/s @ 1.7 g/cm3 |
| RE factor | 1.05 @ 1.7 g/cm3 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| 219 °C (426 °F; 492 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
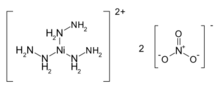
Nickel hydrazine nitrate (NHN), (chemical formula: [Ni(N2H4)3](NO3)2 is an energetic material having explosive properties in between that of primary explosive and a secondary explosive.[1] It is a salt of a coordination compound of nickel with a reaction equation of 3N2H4·H2O + Ni(NO3)2 →〔Ni(N2H4)3〕(NO3)2 + 3H2O[2]
Properly made (talcum powder like consistency)[3]
Preparation
NHN can be synthesized by reacting nickel(II) nitrate hexahydrate with a dilute aqueous solution of hydrazine monohydrate at 65 C.[4] To help speed the drying of the product after filtration from the hot water, it can be rinsed with alcohol. The product is a fluffy powder (density=0.9 g/cm3). To increase its bulk density to (1.2 g/cm3), dextrin in the amount of (1%) of the weight of the nickel(II) nitrate hexahydrate can be added.[5]
Non-primary explosive detonator (NPED)
The sensitivity of NHN straddles the line between highly sensitive primaries and a sensitive secondary, so it can be considered a true non-primary explosive detonator (NPED).
Another benefit of NHN is that it will make the DDT (deflagration to detonation transition) in a cardboard shell, eliminating the danger of shrapnel from a metal shell.
Safety
NHN straddles the line between primary and secondary. Because of this it is a relatively safe explosive to work with having 80x less sensitivity to friction (16.0 N) than lead azide (0.1N) as shown in table 2.
Friction sensitivities of some traditional explosives (lead azide – 0.1N; lead styphnate – 1.5 N; mercury fulminate (white) – 5.0 N; tetrazene – 8.0 N; PETN – 60 N; RDX – 120 N; HMX – 120 N, show that NHN is not very sensitive, and is thereby not exceedingly hazardous in handling.[6]
Table 1. General and structural properties of Nickel hydrazine nitrate[1]
| Molecular formula | Ni H12 N8 O6 |
| Formula weight | 278.69 |
| Color | Purple Violet |
| Crystal density (g/cm3) | 2.1 |
| Average particle size (μm) | 13 |
| Nickel content (%) | 21.16 (21.06) a |
| Hydrazine content (%) | 34.46 (34.45) a |
| Nitrate content (%) | 44.47 (44.49) a |
| Nitrogen content in coordination sphere (%) | 30.25 (30.14) a |
| FTIR peaks, (cm−1) | 3238, 1630 (NH2); 1356,1321 (-NO3) |
| Moisture content (at 333 K for 10 min) (%) | 0.34 |
| Average mol wt of combustion products | 27.35 |
| Percent condensable Ni (l) | 18 |
| Oxygen-fuel ratio | 0.8571 |
| Oxygen balance % | -5.74 |
a Values in brackets are theoretical
Table 2. Comparative properties of Nickel hydrazine nitrate, lead azide and lead styphnate[1]
| Property | Nickel hydrazine nitrate a | Lead azide b | Lead styphnate |
|---|---|---|---|
| Crystal Density (g/cm3) | 2.129 | 4.38 | 3.02 |
| Oxygen balance (%) | – 5.74 c | – 5.50 | -19.00 |
| Heat of combustion (kJ/kg) | 5225 | 2635 | 5234 |
| Heat of formation (kJ/mol) | – 449 | 469 | -385 |
| Heat of explosion (kJ/kg) | 4390 | 1610 | 1912 |
| Pressure output in closed vessel (100 mg in 48 cm3) (kg/cm2) | 17.5 | 8.2 c | |
| Onset of decomposition (K) | 505.7 | 463 | 533.15 |
| Peak of decomposition (K) | 506.5 | 618 | 583.15 |
| Friction sensitivity (kg f) | 1.6 | 0.02 | .15 |
| Impact sensitivity (cm, 400 g wt, 20 mg sample, 50% explosion) | 21 b | 10.5 | 11 |
| ESD sensitivity (J) | 0.02 b | 0.004 | .0002 |
| Vol. of detonation gases (ml/g) | 884 c | 308 | 368 |
| Detonation temperature (K) | 2342 c | 5600 | |
| Detonation pressure (GPa) | 20.8 c (1.7 g/cm3) | 16.1 (3.0 g/cm3) | |
| Detonation velocity (m/s) | 7000 b (1.7 g/cm3) | 4630 (3.0 g/cm3) | 5200 (2.9 g/cm3) |
| RE Factor | 1.05 b (1.7 g/cm3) | .8 (3.0 g/cm3) |
a Experimental value, b literature value, and c theoretical value
References
- ↑ 1.0 1.1 1.2 Hariharanath, B.; Chandrabhanu, K. S.; Rajendran, A. G.; Ravindran, M.; Kartha, C. B. (2006). "Detonator using Nickel Hydrazine Nitrate as Primary Explosive". Defence Science Journal 56 (3): 383–9. doi:10.14429/dsj.56.1904.
- ↑ Xiang, Dong; Zhu, Weihua (15 February 2018). "Thermal decomposition of energetic MOFs nickel hydrazine nitrate crystals from an ab initio molecular dynamics simulation". Computational Materials Science 143: 170–181. doi:10.1016/j.commatsci.2017.11.006.
- ↑ Nickel Hydrazine Nitrate (Dextrinated) https://www.youtube.com/watch?v=rPxdDSUGxo4&t=11s
- ↑ Chhabra, J.S; Talawar, M.B; Makashir, P.S; Asthana, S.N; Singh, Haridwar (2003). "Synthesis, characterization and thermal studies of (Ni/Co) metal salts of hydrazine: Potential initiatory compounds". Journal of Hazardous Materials 99 (3): 225–39. doi:10.1016/S0304-3894(02)00247-9. PMID 12758009.
- ↑ Talawar, M B; Agrawal, A P; Chhabra, J S; Ghatak, C K; Asthana, S N; Rao, K U B (August 2004). "Studies on nickel hydrazinium nitrate (NHN) and bis-(5-nitro-2H tetrazolato-N2)tetraamino cobalt(III) perchlorate (BNCP): Potential lead-free advanced primary explosives". Journal of Scientific & Industrial Research 63 (8): 677–681. http://pyrobin.com/files/Nickelhydrazinnitrat2.pdf.
- ↑ Wojewódka, Andrzej; Bełzowski, Janusz (2011). "Hydrazynowe kompleksy metali przejściowych jako perspektywiczne materiały wybuchowe". Chemik 65 (1): 20–27. http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-BPP2-0011-0002.
 |




