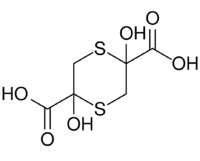
Chemistry:Sulfanegen
| |
| Names | |
|---|---|
| IUPAC name
2,5-Dihydroxy-1,4-dithiane-2,5-dicarboxylic acid
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H8O6S2 | |
| Molar mass | 240.24 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sulfanegen is an experimental antidote for cyanide poisoning.[1] It is being studied as a prodrug for 3-mercaptopyruvic acid (3-MP). 3-MP has been studied as a potential treatment for cyanide poisoning, but the half-life is too short for it to be clinically effective.[2] Instead, alternative chemicals such as sulfanegen, the hemithioacetal cyclic dimer of 3-MP, are being evaluated that produce 3-MP in vivo to compensate for the short half-life of 3-MP itself.[3]
Sulfanegen has been shown to be effective in animal studies.[4] It is being studied as the disodium salt, sulfanegen sodium,[3][5] and the triethanolamine salt, sulfanegen TEA.[6] One advantage various sulfanegen formulations have over existing treatments for acute cyanide poisoning is that they might be administered by intramuscular injection or orally[1] rather than by intravenous infusion.[6]
References
- ↑ 1.0 1.1 "Scientists Discover Fast-Acting Cyanide Antidote". Medgadget. Dec 27, 2007. http://www.medgadget.com/2007/12/scientists_discover_fastacting_cyanide_antidote.html. Retrieved 2015-07-12.
- ↑ Nagahara, N; Li, Q; Sawada, N (2003). "Do antidotes for acute cyanide poisoning act on mercaptopyruvate sulfurtransferase to facilitate detoxification?". Current Drug Targets. Immune, Endocrine and Metabolic Disorders 3 (3): 198–204. doi:10.2174/1568008033340162. PMID 12871026.
- ↑ 3.0 3.1 Brenner, M; Kim, JG; Lee, J; Mahon, SB; Lemor, D; Ahdout, R; Boss, GR; Blackledge, W et al. (2010). "Sulfanegen sodium treatment in a rabbit model of sub-lethal cyanide toxicity". Toxicology and Applied Pharmacology 248 (3): 269–76. doi:10.1016/j.taap.2010.08.002. PMID 20705081.
- ↑ Chan, A; Crankshaw, DL; Monteil, A; Patterson, SE; Nagasawa, HT; Briggs, JE; Kozocas, JA; Mahon, SB et al. (2011). "The combination of cobinamide and sulfanegen is highly effective in mouse models of cyanide poisoning". Clinical Toxicology 49 (5): 366–73. doi:10.3109/15563650.2011.584879. PMID 21740135.
- ↑ Belani, KG; Singh, H; Beebe, DS; George, P; Patterson, SE; Nagasawa, HT; Vince, R (2012). "Cyanide toxicity in juvenile pigs and its reversal by a new prodrug, sulfanegen sodium". Anesthesia and Analgesia 114 (5): 956–61. doi:10.1213/ANE.0b013e31824c4eb5. PMID 22392971.
- ↑ 6.0 6.1 "New Antidote for Cyanide Found". Yahoo News. February 1, 2013. https://news.yahoo.com/antidote-cyanide-found-132827160.html.
 |

