Chemistry:Thiadiazoles
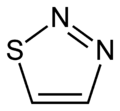
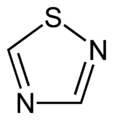
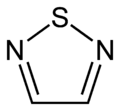
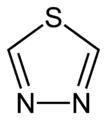
In chemistry thiadiazoles are a sub-family of azole compounds, with the name thiadiazole originating from the Hantzsch–Widman nomenclature. Structurally, they are five-membered heterocyclic compounds containing one sulfur and two nitrogen atoms. They are aromatic ring by virtue of their two double bonds and the sulfur lone pair. Four possible structures exist depending on the relative positions of the heteroatoms; these forms do not interconvert and hence are structural isomers and not tautomers. The compounds themselves are rarely synthesized and possess no particular application, however, compounds bearing them as a structural motif are fairly common in pharmacology. 1,3,4-thiadiazole is the most common, appearing in such medications as cephazolin and acetazolamide.[1][2][3]
References
- ↑ Hu, Yang; Li, Cui-Yun; Wang, Xiao-Ming; Yang, Yong-Hua; Zhu, Hai-Liang (2014). "1,3,4-Thiadiazole: Synthesis, Reactions, and Applications in Medicinal, Agricultural, and Materials Chemistry". Chemical Reviews 114 (10): 5572–5610. doi:10.1021/cr400131u. ISSN 0009-2665. PMID 24716666.
- ↑ Jain, Abhishek Kumar; Sharma, Simant; Vaidya, Ankur; Ravichandran, Veerasamy; Agrawal, Ram Kishore (2013). "1,3,4-Thiadiazole and its Derivatives: A Review on Recent Progress in Biological Activities". Chemical Biology & Drug Design 81 (5): 557–576. doi:10.1111/cbdd.12125. ISSN 1747-0277. PMID 23452185.

- ↑ Wim Dehaen; Vasiliy A. Bakulev; Edward C. Taylor; Jonathan A. Ellman (27 April 2004). The Chemistry of Heterocyclic Compounds, The Chemistry of 1,2,3-Thiadiazoles. John Wiley & Sons. pp. 5–. ISBN 978-0-471-65691-3. https://books.google.com/books?id=-XMt2cdjIfAC&pg=PR5.
 |