Chemistry:Urea perchlorate
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
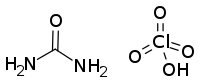
| CO(NH2)2·HClO4 | |
| Molar mass | 159.51 g/mol |
| Appearance | Crystals |
| Melting point | 83 °C (181 °F; 356 K) |
| Large solubility in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Urea perchlorate is a sheet-shaped crystallite with good chemical stability and strong hygroscopicity. It has usage as an oxidizer in liquid explosives[1] including underwater blasting.[2]
Synthesis
The compound is synthesized by gradual addition of urea into a perchloric acid solution:
- CO(NH2)2 + HClO4 → CO(NH2)2·HClO4
An alternative route is addition of urea to hydrochloric acid solution, followed by addition of sodium perchlorate, and filtration of the salt.
- NaClO4·H2O + CO(NH2)2 + HCl → CO(NH2)2·HClO4 + NaCl + H2O
References
- ↑ Liu, Jiping (2015). Liquid Explosives. Springer. p. 6. ISBN 9783662458464.
- ↑ Kusakabe, Masao; Shuzo Fuziwara & Kazuo Shiino, "Underwater blasting method and explosives and devices used therein", US patent 3952655, published 1976-04-27
 |

