Physics:Quantum excitation (accelerator physics)
Quantum excitation is the effect in circular accelerators or storage rings whereby the discreteness of photon emission causes the charged particles (typically electrons) to undergo a random walk or diffusion process.
Mechanism
An electron moving through a magnetic field emits radiation called synchrotron radiation. The expected amount of radiation can be calculated using the classical power. Considering quantum mechanics, however, this radiation is emitted in discrete packets of photons. For this description, the distribution of number of emitted photons and also the energy spectrum for the electron should be determined instead.
In particular, the normalized power spectrum emitted by a charged particle moving in a bending magnet is given by
- [math]\displaystyle{ S(\xi)=\frac{9\sqrt{3}}{8\pi}\xi\int_\xi^\infty K_{5/3}(\bar \xi) d\bar \xi }[/math]
This result has been derived originally by Dmitri Ivanenko and Arseny Sokolov and independently by Julian Schwinger in 1949 [1]
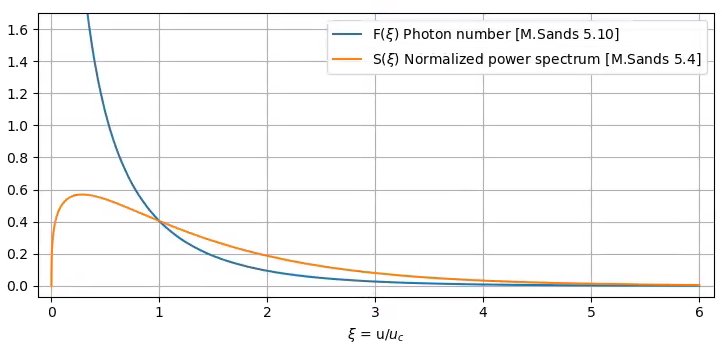
By dividing each power of this power spectrum by the energy, we obtain the photon flux
- [math]\displaystyle{ F(\xi)=\frac{1}{\xi}S(\xi)=\frac{9\sqrt{3}}{8\pi}\int_\xi^\infty K_{5/3}(\bar \xi) d\bar \xi }[/math]
The photon flux from this normalized power spectrum (of all energies) is then
- [math]\displaystyle{ \dot N_{norm} =\frac{9\sqrt{3}}{8\pi}\int_{\xi=0}^\infty\int_{\bar \xi=\xi}^\infty K_{5/3}(\bar \xi) d\bar \xi d\xi = \Gamma(11/6)\Gamma(1/6)\frac{9\sqrt{3}}{8\pi} = \frac{15\sqrt{3}}{8} }[/math]
Note: The fact that the above photon flux integral is finite implies a discrete photon emission, it is a Poisson process. The emission rate is
- [math]\displaystyle{ r_{\gamma} = \frac{5\sqrt{3}}{6} \frac{r_e e_0 \beta^4 \gamma }{ \hbar|\rho| } }[/math] [photon/sec] from [4.4][2] [5.9][2] [5.12][2]
For a travelled distance [math]\displaystyle{ \Delta s }[/math] at a speed close to [math]\displaystyle{ c }[/math] ([math]\displaystyle{ \beta \approx 1 }[/math]), the average number of emitted photons by the particle can be expressed as
- [math]\displaystyle{ \langle n_{\gamma} \rangle = \frac{5\sqrt{3}}{6} \frac{r_e e_0 \gamma }{ \hbar|\rho| } \frac{\Delta s}{c} = \frac{5\sqrt{3}}{6} \frac{\alpha \gamma }{ |\rho| }\Delta s }[/math] where [math]\displaystyle{ \alpha }[/math] is the fine-structure constant
The probability that k photons are emitted over [math]\displaystyle{ \Delta s }[/math] is
- [math]\displaystyle{ Pr \left( n_{\gamma} = k \right) = \frac{\langle n_{\gamma} \rangle^k}{k!} e^{-\langle n_{\gamma} \rangle} }[/math]
The point where the photon number curve and the power spectrum curve are crossing is called the critical energy
- [math]\displaystyle{ u_c=\frac{3c\hbar\gamma^3}{2\rho} }[/math]
where [math]\displaystyle{ \gamma = E/e_0 }[/math],E is the total energy of the charged particle, [math]\displaystyle{ \rho }[/math] is the radius of curvature, [math]\displaystyle{ r_e }[/math] the classical electron radius, [math]\displaystyle{ e_0 = m_e c^2 }[/math] the particle rest mass energy, [math]\displaystyle{ \hbar }[/math] the reduced Planck constant and [math]\displaystyle{ c }[/math] the speed of light.
The mean of the quantum energy is given by [math]\displaystyle{ \langle u\rangle = \frac{8}{15\sqrt{3}}u_c }[/math] and impact mainly the radiation damping. However, the particle motion perturbation (diffusion) is mainly related by the variance of the quantum energy [math]\displaystyle{ \langle u^2\rangle }[/math] and leads to an equilibrium emittance. The diffusion coefficient at a given position [math]\displaystyle{ s }[/math] is given by
- [math]\displaystyle{ d(s) = \frac{55}{48\sqrt{3}}\alpha \left(\frac{\hbar}{m_e c}\right)^2 \frac{\gamma^5}{|\rho(s)|^3} }[/math] [3]
Further reading
For an early analysis of the effect of quantum excitation on electron beam dynamics in storage rings, see the article by Matt Sands.[2]
References
- ↑ Schwinger, Julian (1949) (in en). Qn the Classical Radiation of Accelerated Electrons. https://journals.aps.org/pr/pdf/10.1103/PhysRev.75.1912.
- ↑ 2.0 2.1 2.2 2.3 Sands, Matthew (1970). The Physics of Electron Storage Rings: An Introduction by Matt Sands. http://slac.stanford.edu/pubs/slacreports/reports02/slac-r-121.pdf.
- ↑ Carmignani, Nicola; Nash, Boaz (2014) (in en). Quantum Diffusion Element in AT. https://github.com/atcollab/atdoc/blob/master/QuantumDiffusionElement/qdifElemAT.pdf.
 |