Chemistry:N,N-Dimethylaminomethylferrocene
From HandWiki
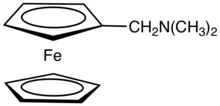
| |
| Names | |
|---|---|
| IUPAC name
N,N-Dimethylaminomethylferrocene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C13H17FeN | |
| Molar mass | 243.131 g·mol−1 |
| Appearance | orange oil |
| Melting point | 5–8 °C (41–46 °F; 278–281 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N,N-Dimethylaminomethylferrocene is the dimethylaminomethyl derivative of ferrocene, (C5H5)Fe(C5H4CH2N(CH3)2. It is an air-stable, dark-orange syrup that is soluble in common organic solvents. The compound is prepared by the reaction of ferrocene with formaldehyde and dimethylamine:[1]
- (C5H5)2Fe + CH2O + HN(CH3)2 → (C5H5)Fe(C5H4CH2N(CH3)2 + H2O
It is a precursor to prototypes of ferrocene-containing redox sensors[2] and diverse ligands.[3]
The amine can be quaternized,[4] which provides access to many derivatives.[5]
References
- ↑ Lednicer, Daniel; Hauser, Charles R. (1960). "N,N-Dimethylaminomethylferrocene Methiodide". Organic Syntheses 40: 31. doi:10.15227/orgsyn.040.0031.
- ↑ Cass, Anthony E. G.; Davis, Graham.; Francis, Graeme D.; Hill, H. Allen O.; Aston, William J.; Higgins, I. John.; Plotkin, Elliot V.; Scott, Lesley D. L. et al. (1984). "Ferrocene-mediated enzyme electrode for amperometric determination of glucose". Analytical Chemistry 56 (4): 667–671. doi:10.1021/ac00268a018. PMID 6721151.
- ↑ Gao, De-Wei; Shi, Yan-Chao; Gu, Qing; Zhao, Zheng-Le; You, Shu-Li (2013). "Enantioselective Synthesis of Planar Chiral Ferrocenes via Palladium-Catalyzed Direct Coupling with Arylboronic Acids". Journal of the American Chemical Society 135 (1): 86–89. doi:10.1021/ja311082u. PMID 23253097.
- ↑ Lednicer, Daniel; Mashburn Jr., T. Arthur; Hauser, Charles R. (1960). "Hydroxymethylferrocene". Organic Syntheses 40: 52. doi:10.15227/orgsyn.040.0052.
- ↑ Lednicer, Daniel; Hauser, Charles R. (1960). "Ferrocenylacetonitrile". Organic Syntheses 40: 45. doi:10.15227/orgsyn.040.0045.
 |

