Biology:Aspartate receptor
| Tar ligand binding domain homologue | |||||||||
|---|---|---|---|---|---|---|---|---|---|
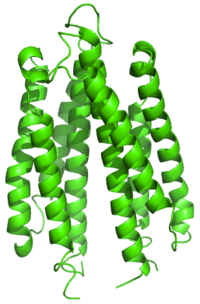 Ribbon diagram of the S. typhimurium aspartate receptor ligand binding domain[1] | |||||||||
| Identifiers | |||||||||
| Symbol | TarH | ||||||||
| Pfam | PF02203 | ||||||||
| InterPro | IPR003122 | ||||||||
| SMART | TarH | ||||||||
| SCOP2 | 1lih / SCOPe / SUPFAM | ||||||||
| CDD | cd00181 | ||||||||
| |||||||||
The aspartate receptor, Tar, is a member of a family of transmembrane receptors that mediate chemotactic response in certain enteric bacteria, such as Salmonella typhimurium and Escherichia coli.[2] These methyl-accepting chemotaxis receptors are one of the first components in the sensory excitation and adaptation responses in bacteria, which act to alter swimming behaviour upon detection of specific chemicals. The aspartate receptor mediates movement towards the attractants aspartate and maltose, and away from the repellents nickel and cobalt. There are many different types of bacterial 60 kDa transmembrane receptors, which share similar topology and signalling mechanisms. They possess three domains: a periplasmic ligand-binding domain, two transmembrane segments, and a cytoplasmic domain. The structure of the ligand-binding domain comprises a closed or partly opened, four-helical bundle with a left-handed twist. The difference in the sequence of the ligand-binding domain between receptors reflects the different ligand specificities. Binding of the ligand causes a conformational change that is transmitted across the membrane to the cytoplasmic activation domain.[3]
References
- ↑ PDB: 1VLT; "High-resolution structures of the ligand binding domain of the wild-type bacterial aspartate receptor". J Mol Biol 262: 186–201. 1996. doi:10.1006/jmbi.1996.0507. PMID 8831788.; rendered with PyMOL
- ↑ "High-resolution structures of the ligand binding domain of the wild-type bacterial aspartate receptor". J. Mol. Biol. 262 (2): 186–201. 1996. doi:10.1006/jmbi.1996.0507. PMID 8831788.
- ↑ "Propagating conformational changes over long (and short) distances in proteins". Proc. Natl. Acad. Sci. U.S.A. 98 (17): 9517–9520. 2001. doi:10.1073/pnas.161239298. PMID 11504940. PMC 55484. https://works.bepress.com/edward_yu/3/download/.

