Biology:CRISPR RNA
CRISPR RNA or crRNA is a RNA transcript from the CRISPR locus.[1] CRISPR-Cas (clustered, regularly interspaced short palindromic repeats - CRISPR associated systems) is an adaptive immune system found in bacteria and archaea to protect against mobile genetic elements, like viruses, plasmids, and transposons.[2] The CRISPR locus contains a series of repeats interspaced with unique spacers. These unique spacers can be acquired from MGEs.[2]
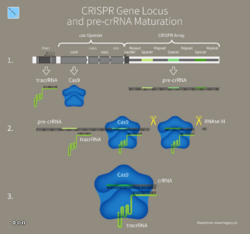
Pre-crRNA is formed after the transcription of the CRISPR locus and before being processed by Cas proteins. Mature crRNA transcripts contain a partial conserved section of repeat and a sequence of spacer that is complementary to the target DNA.[3] crRNA forms an effector complex with a single nuclease or multiple Cas proteins called a Cascade (CRISPR-associated complex for antiviral defense).[3][1] Once the effector complex is formed a Cas nuclease or single effector protein will cause interference guided by the crRNA match.[4]
Function
Type-I
Type-I CRISPR systems are characterized by Cas3, a nuclease-helicase protein, and the multi-subunit Cascade (CRISPR-associated complex for antiviral defense). The crRNA can form a complex with the Cas proteins in the Cascade and guide the complex to the target DNA sequence. Cas3 is recruited for the nuclease-helicase activity.[5]
Typically in the Cascade, Cas6 generates the mature crRNAs while Cas5 and Cas7 process and stabilize the crRNA.[6]
Type-II
Type-II CRISPR systems[7] are characterized by the single signature nuclease Cas9.[8] In type-II CRISPR systems crRNA and tracrRNA (trans-activating CRISPR RNA) can form a complex known as the guide RNA or gRNA.[9] The crRNA within the gRNA is what matches up with the target sequence or protospacer after the PAM is found. Once the match is made Cas9 will make a double-stranded break.
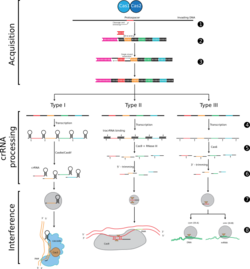
Type-III
Type-III CRISPR systems are characterized by Cas10, an RNA cleaving protein.[10] Similar to type-I, a large subunit effector complex is formed and crRNA guides the complex to the target sequence. Cas6 helps to generate the mature crRNA.[10]
Type-IV
Type-IV CRISPR systems do not have an effector nuclease and are associated with plasmids and prophages. A Cas6-like enzyme is associated with the maturation of the crRNA. Not all type-IV systems have a CRISPR locus and therefore do not have crRNA.[11]
Type-V
Type-V CRISPR systems are characterized by Cas12, a nuclease that can cleave ssDNA, dsDNA, and RNA.[7] Like Cas9, Cas12 is the single effector nuclease. Type-V systems process pre-crRNA without tracrRNA. The mature crRNA in complex with Cas12 target the DNA sequence of interest and cleave the DNA.[12]
Type-VI
Type-VI CRISPR systems are characterized by Cas13, a single effector protein that targets RNA. Like the type-V system, Cas13 can process the pre-crRNA without tracrRNA. The mature crRNA in complex with Cas13 guides the complex to the target RNA and degrades it.[13]
References
- ↑ 1.0 1.1 Gasiunas, Giedrius; Barrangou, Rodolphe; Horvath, Philippe; Siksnys, Virginijus (2012-09-25). "Cas9–crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria" (in en). Proceedings of the National Academy of Sciences 109 (39): E2579-86. doi:10.1073/pnas.1208507109. ISSN 0027-8424. PMID 22949671.
- ↑ 2.0 2.1 Faure, Guilhem; Shmakov, Sergey A.; Yan, Winston X.; Cheng, David R.; Scott, David A.; Peters, Joseph E.; Makarova, Kira S.; Koonin, Eugene V. (August 2019). "CRISPR–Cas in mobile genetic elements: counter-defence and beyond" (in en). Nature Reviews Microbiology 17 (8): 513–525. doi:10.1038/s41579-019-0204-7. ISSN 1740-1534. https://www.nature.com/articles/s41579-019-0204-7.
- ↑ 3.0 3.1 Karvelis, Tautvydas; Gasiunas, Giedrius; Miksys, Algirdas; Barrangou, Rodolphe; Horvath, Philippe; Siksnys, Virginijus (2013-05-01). "crRNA and tracrRNA guide Cas9-mediated DNA interference in Streptococcus thermophilus". RNA Biology 10 (5): 841–851. doi:10.4161/rna.24203. ISSN 1547-6286. PMID 23535272.
- ↑ Jinek, Martin; Chylinski, Krzysztof; Fonfara, Ines; Hauer, Michael; Doudna, Jennifer A.; Charpentier, Emmanuelle (2012-08-17). "A programmable dual RNA-guided DNA endonuclease in adaptive bacterial immunity". Science 337 (6096): 816–821. doi:10.1126/science.1225829. ISSN 0036-8075. PMID 22745249. Bibcode: 2012Sci...337..816J.
- ↑ Sinkunas, Tomas; Gasiunas, Giedrius; Fremaux, Christophe; Barrangou, Rodolphe; Horvath, Philippe; Siksnys, Virginijus (2011-04-06). "Cas3 is a single-stranded DNA nuclease and ATP-dependent helicase in the CRISPR/Cas immune system". The EMBO Journal 30 (7): 1335–1342. doi:10.1038/emboj.2011.41. ISSN 0261-4189. PMID 21343909.
- ↑ Brendel, Jutta; Stoll, Britta; Lange, Sita J.; Sharma, Kundan; Lenz, Christof; Stachler, Aris-Edda; Maier, Lisa-Katharina; Richter, Hagen et al. (2014-03-07). "A Complex of Cas Proteins 5, 6, and 7 Is Required for the Biogenesis and Stability of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-derived RNAs (crRNAs) in Haloferax volcanii". The Journal of Biological Chemistry 289 (10): 7164–7177. doi:10.1074/jbc.M113.508184. ISSN 0021-9258. PMID 24459147.
- ↑ 7.0 7.1 Makarova, Kira S.; Wolf, Yuri I.; Iranzo, Jaime; Shmakov, Sergey A.; Alkhnbashi, Omer S.; Brouns, Stan J. J.; Charpentier, Emmanuelle; Cheng, David et al. (February 2020). "Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants" (in en). Nature Reviews Microbiology 18 (2): 67–83. doi:10.1038/s41579-019-0299-x. ISSN 1740-1534. PMID 31857715.
- ↑ Heler, Robert; Samai, Poulami; Modell, Joshua W.; Weiner, Catherine; Goldberg, Gregory W.; Bikard, David; Marraffini, Luciano A. (2015-03-12). "Cas9 specifies functional viral targets during CRISPR-Cas adaptation". Nature 519 (7542): 199–202. doi:10.1038/nature14245. ISSN 0028-0836. PMID 25707807. Bibcode: 2015Natur.519..199H.
- ↑ Charpentier, Emmanuelle; Richter, Hagen; van der Oost, John; White, Malcolm F. (2015-05-01). "Biogenesis pathways of RNA guides in archaeal and bacterial CRISPR-Cas adaptive immunity". FEMS Microbiology Reviews 39 (3): 428–441. doi:10.1093/femsre/fuv023. ISSN 0168-6445. PMID 25994611.
- ↑ 10.0 10.1 Kolesnik, Matvey V.; Fedorova, Iana; Karneyeva, Karyna A.; Artamonova, Daria N.; Severinov, Konstantin V. (2021-10-01). "Type III CRISPR-Cas Systems: Deciphering the Most Complex Prokaryotic Immune System" (in en). Biochemistry (Moscow) 86 (10): 1301–1314. doi:10.1134/S0006297921100114. ISSN 1608-3040. PMID 34903162. PMC 8527444. https://doi.org/10.1134/S0006297921100114.
- ↑ Pinilla-Redondo, Rafael; Mayo-Muñoz, David; Russel, Jakob; Garrett, Roger A.; Randau, Lennart; Sørensen, Søren J.; Shah, Shiraz A. (2020-02-28). "Type IV CRISPR-Cas systems are highly diverse and involved in competition between plasmids". Nucleic Acids Research 48 (4): 2000–2012. doi:10.1093/nar/gkz1197. ISSN 1362-4962. PMID 31879772.
- ↑ Paul, Bijoya; Montoya, Guillermo (February 2020). "CRISPR-Cas12a: Functional overview and applications". Biomedical Journal 43 (1): 8–17. doi:10.1016/j.bj.2019.10.005. ISSN 2319-4170. PMID 32200959.
- ↑ O'Connell, Mitchell R. (2019-01-04). "Molecular Mechanisms of RNA Targeting by Cas13-containing Type VI CRISPR-Cas Systems". Journal of Molecular Biology 431 (1): 66–87. doi:10.1016/j.jmb.2018.06.029. ISSN 1089-8638. PMID 29940185. https://pubmed.ncbi.nlm.nih.gov/29940185/.
 |
