Biology:FAM83H
 Generic protein structure example |
FAM83H is a gene in humans that encodes a protein known as FAM83H (uncharacterized protein FAM83H). FAM83H is targeted for the nucleus and it predicted to play a role in the structural development and calcification of tooth enamel.
Gene
Location
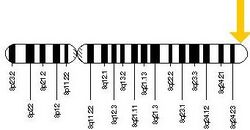
FAM83H is located on the long arm of chromosome 8 (8q24.3), starting at 143723933 and ending at 143738030. The FAM83H gene spans 14097 base pairs and is orientated on the—strand. The coding region is made up of 5,604 base pairs and 5 exons.[1]
Expression
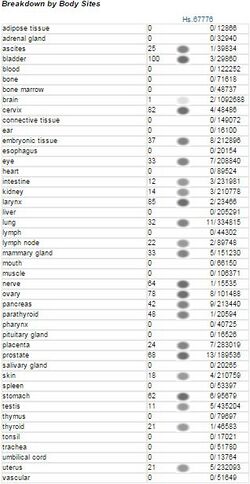
FAM83H is ubiquitously expressed throughout the human body at relatively low levels.[2][3]
Transcript Variants
In humans, there is only one known major product of the FAM83H gene.[4][5][6]
Homology
Paralogs
There are no paralogs of FAM83H[7]
Orthologs
Below is a table of a variety of orthologs of the human FAM83H. The table include closely, intermediately and distantly related orthologs.
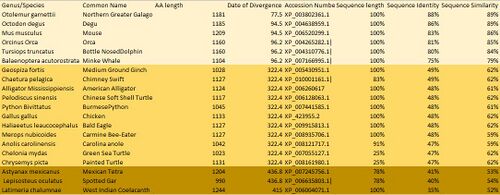
Orthologs of the human protein FAM83H are listed above in descending order or date of divergence and then ascending order of percent identity. FAM83H is highly conserved throughout all orthologs, this is demonstrated with a 40% identity in the least similar ortholog. FAM83H has evolved slowly and evenly over time.[8][9]
Protein
General Properties
The molecular weight of FAM83H is 127.1kD and contains 1179 amino acids. The isoelectric point is 6.52. There are no significant positive or negative charge clusters in the protein. There is a stretch of 21 0’s from 254-275 and a stretch of 24 0’s from 420-444.1 [10]
Composition
FAM83H is proline rich, being 10.32% protein, and is asparagine deficient with only 1.1%. The percent composition of each amino acid is fairly consistent throughout the orthologs of the protein. The most distant ortholog displays the most variance in amino acid composition.
Domains
FAM83H has two known domains. The PLDc_FAM83H (phospholipase like domain) domain stretches from 17-281 on FAM83H. It lacks the functionally important histidine, so while it may share similar structure it most likely lacks PLD activity. The MIP-T3 microtubule binding domain stretches from 909-1176.[11]
Post-translational modifications
FAM83H is highly phosphorylated post modification. There are 11 predicted phosphorylated sites. There are two motifs with high probability of post translational modification sumoylation sites. Sumoylation sites are involved in a number of cellular processes, including nuclear-cytosolic transport, transcriptional regulation and protein stability. FAM83H does not have a signal peptide

Secondary Structure
Fam83H is primarily composed of alpha helices and random coils. Alpha helices comprise the majority of the protein. There is a transmembrane domain from 231-252.[12][13]
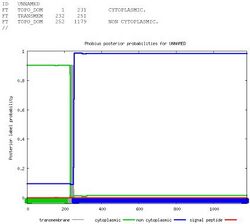
Subcellular Localization
Protein FAM83H is targeted to the nucleus.[14]
Interacting Proteins
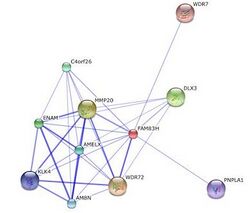
FAM83H was found to interact with WDR72 and MMP20.[15] MMP20 is responsible for the breakdown of extracellular matrix and plays a role in tissue remodeling in ameloblasts. mutations in WDR72 is thought to play a role in amelogenesis imperfecta
Clinical Significance
Disease Association
People who suffer from amelogenesis imperfecta have lost function in FAM83H.[16][17]
References
- ↑ "NCBI gene database". NCBI. https://www.ncbi.nlm.nih.gov/gene/84542.
- ↑ "GEO profiles". NCBI geo profiles. https://www.ncbi.nlm.nih.gov/geoprofiles/2886485.
- ↑ "EST profiles". NCBI EST profiles. https://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist=Hs.468653.
- ↑ "Emsembl". Vega. http://useast.ensembl.org/Homo_sapiens/Transcript/Summary?db=core;g=ENSG00000162929;r=2:61372266-61390298;t=ENST00000398622.
- ↑ "Genecards". The Gene Human Database. https://www.genecards.org/cgi-bin/carddisp.pl?gene=KIAA1841&search=KIAA1841.
- ↑ "Aceview". NCBI. https://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/av.cgi?db=human&term=KIAA1841&submit=Go.
- ↑ "Genecards". The Gene Human Database. https://www.genecards.org/cgi-bin/carddisp.pl?gene=KIAA1841&search=KIAA1841.
- ↑ "BLAST". NCBI. http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE_TYPE=BlastSearch&BLAST_SPEC=OGP__9606__9558.
- ↑ Hedges, SB. "TimeTree". Bioinformatics. http://www.timetree.org/.
- ↑ "SAPS". Statistical Analysis of Protein Sequence, Biology Workbench. http://seqtool.sdsc.edu/CGI/BW.cgi#!.[yes|permanent dead link|dead link}}]
- ↑ "NCBI Structure". The Gene Human Database. https://www.genecards.org/cgi-bin/carddisp.pl?gene=KIAA1841&search=KIAA1841.
- ↑ "PELE". San Diego Supercomputer Center. http://workbench.sdsc.edu/.
- ↑ "CHOFAS (Predict Secondary Structure of PS". Chou-Fasman. http://seqtool.sdsc.edu.
- ↑ "PSORT II". Expasy. http://psort.hgc.jp/form2.html.
- ↑ "IntAct". EMNL-EBI. http://www.ebi.ac.uk/intact/pages/interactions/interactions.xhtml?conversationContext=1.
- ↑ "NCBI gene database". NCBI. https://www.ncbi.nlm.nih.gov/gene/84542.
- ↑ "Genecards". The Gene Human Database. https://www.genecards.org/cgi-bin/carddisp.pl?gene=KIAA1841&search=KIAA1841.
Further reading
- "FAM83H mutations in families with autosomal-dominant hypocalcified amelogenesis imperfecta". Am. J. Hum. Genet. 82 (2): 489–94. 2008. doi:10.1016/j.ajhg.2007.09.020. PMID 18252228.
- "Fam83h is associated with intracellular vesicles and ADHCAI". J. Dent. Res. 88 (11): 991–6. 2009. doi:10.1177/0022034509349454. PMID 19828885.
- "Novel FAM83H mutations in Turkish families with autosomal dominant hypocalcified amelogenesis imperfecta". Clin. Genet. 75 (4): 401–4. 2009. doi:10.1111/j.1399-0004.2008.01112.x. PMID 19220331.
- "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. 1996. doi:10.1101/gr.6.9.791. PMID 8889548.
- "Mutational spectrum of FAM83H: the C-terminal portion is required for tooth enamel calcification". Hum. Mutat. 29 (8): E95–9. 2008. doi:10.1002/humu.20789. PMID 18484629.
- "Ultrastructural analyses of deciduous teeth affected by hypocalcified amelogenesis imperfecta from a family with a novel Y458X FAM83H nonsense mutation". Cells Tissues Organs (Print) 191 (3): 235–9. 2010. doi:10.1159/000252801. PMID 20160442.
- "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. 2002. doi:10.1073/pnas.242603899. PMID 12477932.
- "Transcriptome characterization elucidates signaling networks that control human ES cell growth and differentiation". Nat. Biotechnol. 22 (6): 707–16. 2004. doi:10.1038/nbt971. PMID 15146197.
- "Phenotypic variation in FAM83H-associated amelogenesis imperfecta". J. Dent. Res. 88 (4): 356–60. 2009. doi:10.1177/0022034509333822. PMID 19407157.
- "Identification of a novel FAM83H mutation and microhardness of an affected molar in autosomal dominant hypocalcified amelogenesis imperfecta". Int Endod J 42 (11): 1039–43. 2009. doi:10.1111/j.1365-2591.2009.01617.x. PMID 19825039.
