Biology:Filamentation
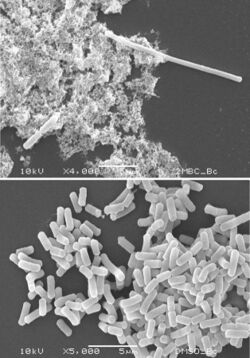
Filamentation is the anomalous growth of certain bacteria, such as Escherichia coli, in which cells continue to elongate but do not divide (no septa formation).[1][2] The cells that result from elongation without division have multiple chromosomal copies.[1]
In the absence of antibiotics or other stressors, filamentation occurs at a low frequency in bacterial populations (4–8% short filaments and 0–5% long filaments in 1- to 8-hour cultures).[3] The increased cell length can protect bacteria from protozoan predation and neutrophil phagocytosis by making ingestion of cells more difficult.[1][3][4][5] Filamentation is also thought to protect bacteria from antibiotics, and is associated with other aspects of bacterial virulence such as biofilm formation.[6][7]
The number and length of filaments within a bacterial population increases when the bacteria are exposed to different physical, chemical and biological agents (e.g. UV light, DNA synthesis-inhibiting antibiotics, bacteriophages).[3][8] This is termed conditional filamentation.[2] Some of the key genes involved in filamentation in E. coli include sulA, minCD and damX.[9][10]
Filament formation
Antibiotic-induced filamentation
Some peptidoglycan synthesis inhibitors (e.g. cefuroxime, ceftazidime) induce filamentation by inhibiting the penicillin binding proteins (PBPs) responsible for crosslinking peptidoglycan at the septal wall (e.g. PBP3 in E. coli and P. aeruginosa). Because the PBPs responsible for lateral wall synthesis are relatively unaffected by cefuroxime and ceftazidime, cell elongation proceeds without any cell division and filamentation is observed.[3][11][12]
DNA synthesis-inhibiting and DNA damaging antibiotics (e.g. metronidazole, mitomycin C, the fluoroquinolones, novobiocin) induce filamentation via the SOS response. The SOS response inhibits septum formation until the DNA can be repaired, this delay stopping the transmission of damaged DNA to progeny. Bacteria inhibit septation by synthesizing protein SulA, an FtsZ inhibitor that halts Z-ring formation, thereby stopping recruitment and activation of PBP3.[3][13] If bacteria are deprived of the nucleobase thymine by treatment with folic acid synthesis inhibitors (e.g. trimethoprim), this also disrupts DNA synthesis and induces SOS-mediated filamentation. Direct obstruction of Z-ring formation by SulA and other FtsZ inhibitors (e.g. berberine) induces filamentation too.[3][14][15]
Some protein synthesis inhibitors (e.g. kanamycin), RNA synthesis inhibitors (e.g. bicyclomycin) and membrane disruptors (e.g. daptomycin, polymyxin B) cause filamentation too, but these filaments are much shorter than the filaments induced by the above antibiotics.[3]
Stress-induced filamentation
Filamentation is often a consequence of environmental stress. It has been observed in response to temperature shocks,[16] low water availability,[17] high osmolarity,[18] extreme pH,[19] and UV exposure.[20] UV light damages bacterial DNA and induces filamentation via the SOS response.[3][21] Starvation can also cause bacterial filamentation.[9] For example, if bacteria are deprived of the nucleobase thymine, this disrupts DNA synthesis and induces SOS-mediated filamentation.[3][22]
Nutrient-induced filamentation
Several macronutrients and biomolecules can cause bacterial cells to filament, including the amino acids glutamine, proline and arginine, and some branched-chain amino acids.[23] Certain bacterial species, such as Paraburkholderia elongata, will also filament as a result of a tendency to accumulate phosphate in the form of polyphosphate, which can chelate metal cofactors needed by division proteins.[2] In addition, filamentation is induced by nutrient-rich conditions in the intracellular pathogen Bordetella atropi. This occurs via the highly conserved UDP-glucose pathway. UDP-glucose biosynthesis and sensing suppresses bacterial cell division, with the ensuing filamentation allowing B. atropi to spread to neighboring cells.[24]
Intrinsic dysbiosis-induced filamentation
Filamentation can also be induced by other pathways affecting thymidylate synthesis. For instance, partial loss of dihydrofolate reductase (DHFR) activity causes reversible filamentation.[25] DHFR has a critical role in regulating the amount of tetrahydrofolate, which is essential for purine and thymidylate synthesis. DHFR activity can be inhibited by mutations or by high concentrations of the antibiotic trimethoprim (see antibiotic-induced filamentation above).
Overcrowding of the periplasm or envelope can also induce filamentation in Gram-negative bacteria by disrupting normal divisome function.[26][27]
Filamentation and biotic interactions
Several examples of filamentation that result from biotic interactions between bacteria and other organisms or infectious agents have been reported. Filamentous cells are resistant to ingestion by bacterivores, and environmental conditions generated during predation can trigger filamentation.[28] Filamentation can also be induced by signalling factors produced by other bacteria.[29] In addition, Agrobacterium spp. filament in proximity to plant roots,[30] and E. coli filaments when exposed to plant extracts.[31] Lastly, bacteriophage infection can result in filamentation via the expression of proteins that inhibit divisome assembly.[8]
See also
- Bacterial morphological plasticity
- Filamentous bacteriophage
- Filamentous cyanobacteria
- Segmented filamentous bacteria
References
- ↑ 1.0 1.1 1.2 "Filamentous Escherichia coli cells swimming in tapered microcapillaries". Research in Microbiology 165 (3): 166–74. April 2014. doi:10.1016/j.resmic.2014.01.007. PMID 24566556.
- ↑ 2.0 2.1 2.2 "Conditional filamentation as an adaptive trait of bacteria and its ecological significance in soils". Environmental Microbiology 24 (1): 1–17. January 2022. doi:10.1111/1462-2920.15871. PMID 34929753.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 "Morphological and ultrastructural changes in bacterial cells as an indicator of antibacterial mechanism of action". Cellular and Molecular Life Sciences 73 (23): 4471–4492. December 2016. doi:10.1007/s00018-016-2302-2. PMID 27392605. https://zenodo.org/record/883501.
- ↑ "Grazing pressure by a bacterivorous flagellate reverses the relative abundance of Comamonas acidovorans PX54 and Vibrio strain CB5 in chemostat cocultures". Applied and Environmental Microbiology 64 (5): 1910–8. May 1998. doi:10.1128/AEM.64.5.1910-1918.1998. PMID 9572971. Bibcode: 1998ApEnM..64.1910H.
- ↑ "Bacterial filament formation, a defense mechanism against flagellate grazing, is growth rate controlled in bacteria of different phyla". Applied and Environmental Microbiology 65 (1): 25–35. January 1999. doi:10.1128/AEM.65.1.25-35.1999. PMID 9872755. Bibcode: 1999ApEnM..65...25H.
- ↑ "Morphological plasticity as a bacterial survival strategy". Nature Reviews. Microbiology 6 (2): 162–8. February 2008. doi:10.1038/nrmicro1820. PMID 18157153.
- ↑ "Role of filamentation in Galleria mellonella killing by Candida albicans". Microbes and Infection 12 (6): 488–96. June 2010. doi:10.1016/j.micinf.2010.03.001. PMID 20223293.
- ↑ 8.0 8.1 "Cryptic-Prophage-Encoded Small Protein DicB Protects Escherichia coli from Phage Infection by Inhibiting Inner Membrane Receptor Proteins". Journal of Bacteriology 201 (23). December 2019. doi:10.1128/JB.00475-19. PMID 31527115.
- ↑ 9.0 9.1 "Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring". Journal of Bacteriology 175 (4): 1118–1125. February 1993. doi:10.1128/jb.175.4.1118-1125.1993. PMID 8432706.
- ↑ "DamX Controls Reversible Cell Morphology Switching in Uropathogenic Escherichia coli". mBio 7 (4). August 2016. doi:10.1128/mBio.00642-16. PMID 27486187.
- ↑ "Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12". Proceedings of the National Academy of Sciences of the United States of America 72 (8): 2999–3003. August 1975. doi:10.1073/pnas.72.8.2999. PMID 1103132. Bibcode: 1975PNAS...72.2999S.
- ↑ "β-Lactams and β-Lactamase Inhibitors: An Overview". Cold Spring Harbor Perspectives in Medicine 6 (8): a025247. August 2016. doi:10.1101/cshperspect.a025247. PMID 27329032.
- ↑ "Crystal structure of the SOS cell division inhibitor SulA and in complex with FtsZ". Proceedings of the National Academy of Sciences of the United States of America 100 (13): 7889–94. June 2003. doi:10.1073/pnas.1330742100. PMID 12808143. Bibcode: 2003PNAS..100.7889C.
- ↑ "Antimicrobial peptide CRAMP (16-33) stalls bacterial cytokinesis by inhibiting FtsZ assembly". Biochemistry 53 (41): 6426–9. October 2014. doi:10.1021/bi501115p. PMID 25294259.
- ↑ "Bacterial cell division as a target for new antibiotics". Current Opinion in Microbiology 16 (5): 522–530. October 2013. doi:10.1016/j.mib.2013.07.006. PMID 23932516.
- ↑ "Behaviours of log phase cultures of eight strains of Escherichia coli incubated at temperatures of 2, 6, 8 and 10 degrees C". International Journal of Food Microbiology 119 (3): 200–206. November 2007. doi:10.1016/j.ijfoodmicro.2007.07.043. PMID 17719669.
- ↑ "Survival and filamentation of Salmonella enterica serovar enteritidis PT4 and Salmonella enterica serovar typhimurium DT104 at low water activity". Applied and Environmental Microbiology 66 (4): 1274–1279. April 2000. doi:10.1128/AEM.66.4.1274-1279.2000. PMID 10742199. Bibcode: 2000ApEnM..66.1274M.
- ↑ "Reduced water availability influences the dynamics, development, and ultrastructural properties of Pseudomonas putida biofilms". Journal of Bacteriology 185 (20): 6199–6204. October 2003. doi:10.1128/JB.185.20.6199-6204.2003. PMID 14526033.
- ↑ "Filament formation by foodborne bacteria under sublethal stress". International Journal of Food Microbiology 165 (2): 97–110. July 2013. doi:10.1016/j.ijfoodmicro.2013.05.001. PMID 23727653.
- ↑ "Ultraviolet radiation induces filamentation in bacterial assemblages from North Andean Patagonian lakes". Photochemistry and Photobiology 86 (4): 871–881. July 2010. doi:10.1111/j.1751-1097.2010.00758.x. PMID 20528974.
- ↑ "Evidence for a relationship between deoxyribonucleic acid metabolism and septum formation in Escherichia coli". Journal of Bacteriology 95 (1): 123–131. January 1968. doi:10.1128/JB.95.1.123-131.1968. PMID 4867214.
- ↑ "Studies of intracellular thymidine nucleotides. Thymineless death and the recovery after re-addition of thymine in Escherichia coli K 12". European Journal of Biochemistry 60 (1): 57–66. December 1975. doi:10.1111/j.1432-1033.1975.tb20975.x. PMID 1107038.
- ↑ "Formation of Filaments by Pseudomonas putida". Applied and Environmental Microbiology 50 (2): 364–372. August 1985. doi:10.1128/aem.50.2.364-372.1985. PMID 16346856. Bibcode: 1985ApEnM..50..364J.
- ↑ "Bacterial filamentation as a mechanism for cell-to-cell spread within an animal host". Nature Communications 13 (1): 693. February 2022. doi:10.1038/s41467-022-28297-6. PMID 35121734. Bibcode: 2022NatCo..13..693T.
- ↑ "Metabolic response to point mutations reveals principles of modulation of in vivo enzyme activity and phenotype". Molecular Systems Biology 17 (6): e10200. June 2021. doi:10.15252/msb.202110200. PMID 34180142.
- ↑ "Cell division defects in Escherichia coli deficient in the multidrug efflux transporter AcrEF-TolC". Journal of Bacteriology 187 (22): 7815–7825. November 2005. doi:10.1128/JB.187.22.7815-7825.2005. PMID 16267305.
- ↑ "Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence". Molecular Microbiology 79 (1): 240–263. January 2011. doi:10.1111/j.1365-2958.2010.07445.x. PMID 21166906.
- ↑ "Direct and indirect effects of protist predation on population size structure of a bacterial strain with high phenotypic plasticity". Applied and Environmental Microbiology 72 (1): 78–86. January 2006. doi:10.1128/AEM.72.1.78-86.2006. PMID 16391028. Bibcode: 2006ApEnM..72...78C.
- ↑ "Interspecies signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa". Molecular Microbiology 68 (1): 75–86. April 2008. doi:10.1111/j.1365-2958.2008.06132.x. PMID 18312265.
- ↑ "Proximity of Agrobacterium to living plant tissues induces conversion to a filamentous bacterial form". Plant Cell Reports 20 (3): 250–255. February 2001. doi:10.1007/s002990100315.
- ↑ "Aqueous extract of Hibiscus sabdariffa inhibits pedestal induction by enteropathogenic E. coli and promotes bacterial filamentation in vitro". PLOS ONE 14 (3): e0213580. 2019. doi:10.1371/journal.pone.0213580. PMID 30849110. Bibcode: 2019PLoSO..1413580M.
 |
