Biology:Hypothalamic–pituitary–thyroid axis

The hypothalamic–pituitary–thyroid axis (HPT axis for short, a.k.a. thyroid homeostasis or thyrotropic feedback control) is part of the neuroendocrine system responsible for the regulation of metabolism and also responds to stress.
As its name suggests, it depends upon the hypothalamus, the pituitary gland, and the thyroid gland.
The hypothalamus senses low circulating levels of thyroid hormone (Triiodothyronine (T3) and Thyroxine (T4)) and responds by releasing thyrotropin-releasing hormone (TRH). The TRH stimulates the anterior pituitary to produce thyroid-stimulating hormone (TSH). The TSH, in turn, stimulates the thyroid to produce thyroid hormone until levels in the blood return to normal. Thyroid hormone exerts negative feedback control over the hypothalamus as well as anterior pituitary, thus controlling the release of both TRH from hypothalamus and TSH from anterior pituitary gland.[2]
The HPA, HPG, and HPT axes are three pathways in which the hypothalamus and pituitary direct neuroendocrine function.
Physiology
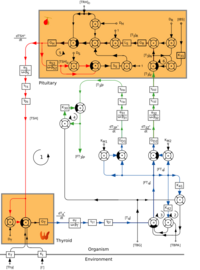
Thyroid homeostasis results from a multi-loop feedback system that is found in virtually all higher vertebrates. Proper function of thyrotropic feedback control is indispensable for growth, differentiation, reproduction and intelligence. Very few animals (e.g. axolotls and sloths) have impaired thyroid homeostasis that exhibits a very low set-point that is assumed to underlie the metabolic and ontogenetic anomalies of these animals.
The pituitary gland secretes thyrotropin (TSH; Thyroid Stimulating Hormone) that stimulates the thyroid to secrete thyroxine (T4) and, to a lesser degree, triiodothyronine (T3). The major portion of T3, however, is produced in peripheral organs, e.g. liver, adipose tissue, glia and skeletal muscle by deiodination from circulating T4. Deiodination is controlled by numerous hormones and nerval signals including TSH, vasopressin and catecholamines.
Both peripheral thyroid hormones (iodothyronines) inhibit thyrotropin secretion from the pituitary (negative feedback). Consequently, equilibrium concentrations for all hormones are attained.
TSH secretion is also controlled by thyrotropin releasing hormone (thyroliberin, TRH), whose secretion itself is again suppressed by plasma T4 and T3 in CSF (long feedback, Fekete–Lechan loop).[4] Additional feedback loops are ultrashort feedback control of TSH secretion (Brokken-Wiersinga-Prummel loop)[5] and linear feedback loops controlling plasma protein binding.
Recent research suggested the existence of an additional feedforward motif linking TSH release to deiodinase activity in humans.[6][7][8] The existence of this TSH-T3 shunt could explain why deiodinase activity is higher in hypothyroid patients and why a minor fraction of affected individuals may benefit from substitution therapy with T3.[9]
Convergence of multiple afferent signals in the control of TSH release including but not limited to T3,[10] cytokines[11][12] and TSH receptor antibodies[13] may be the reason for the observation that the relation between free T4 concentration and TSH levels deviates[14][15][16][17] from a pure loglinear relation that has previously been proposed.[18] Recent research suggests that ghrelin also plays a role in the stimulation of T4 production and the subsequent suppression of TSH directly and by negative feedback.[19]
Functional states of thyrotropic feedback control
- Euthyroidism: Normal thyroid function
- Hypothyroidism: Reduced thyroid function
- primary hypothyroidism: Feedback loop interrupted by low thyroid secretory capacity, e.g. after thyroid surgery or in case of autoimmune thyroiditis
- secondary hypothyroidism: Feedback loop interrupted on the level of pituitary, e.g. in anterior pituitary failure
- tertiary hypothyroidism: Lacking stimulation by TRH, e.g. in hypothalamic failure, Pickardt–Fahlbusch syndrome or euthyroid sick syndrome.
- Hyperthyroidism: Inappropriately increased thyroid function
- primary hyperthyroidism: Inappropriate secretion of thyroid hormones, e.g. in case of Graves' disease.
- secondary hyperthyroidism: Rare condition, e.g. in case of TSH producing pituitary adenoma or partial thyroid hormone resistance.
- Thyrotoxicosis: Over-supply with thyroid hormones, e.g. by overdosed exogenously levothyroxine supplementation.
- Low-T3 syndrome and high-T3 syndrome: Consequences of step-up hypodeiodination, e.g. in critical illness as an example for type 1 allostasis,[20] or hyperdeiodination, as in type 2 allostasis, including posttraumatic stress disorder.[12]
- Resistance to thyroid hormone: Feedback loop interrupted on the level of pituitary thyroid hormone receptors.
Diagnostics
Standard procedures cover the determination of serum levels of the following hormones:
- TSH (thyrotropin, thyroid stimulating hormone)
- Free T4
- Free T3
For special conditions the following assays and procedures may be required:
- Total T4
- Total T3
- TBG
- TRH test
- Thyroid's secretory capacity (GT)[21]
- Sum activity of peripheral deiodinases (GD)[21]
- TSH Index (TSHI)[22]
See also
- Thyroid function tests
- Hypothalamic–pituitary–adrenal axis
- Hypothalamic–pituitary–gonadal axis
- Hypothalamic–neurohypophyseal system
- SimThyr, a free computer simulation for thyroid homeostasis in humans
References
- ↑ References used in overview figure are found in image article in Commons: References.
- ↑ "TSH and Thyrotropic Agonists: Key Actors in Thyroid Homeostasis". Journal of Thyroid Research 2012: 1–29. 2012. doi:10.1155/2012/351864. PMID 23365787.
- ↑ References used in detailed figure are found in image article in Commons: References.
- ↑ Lechan, Ronald M.; Fekete, C (2004). "Feedback regulation of thyrotropin-releasing hormone (TRH): mechanisms for the non-thyroidal illness syndrome". Journal of Endocrinological Investigation 27 (6 Suppl): 105–19. PMID 15481810.
- ↑ "Ultra short-loop feedback control of thyrotropin secretion". Thyroid 14 (10): 825–9. 2004. doi:10.1089/thy.2004.14.825. PMID 15588378.
- ↑ "Homeostatic equilibria between free thyroid hormones and pituitary thyrotropin are modulated by various influences including age, body mass index and treatment". Clinical Endocrinology 81 (6): 907–15. 2014. doi:10.1111/cen.12527. PMID 24953754.
- ↑ Dietrich, JW; Midgley, JE; Larisch, R; Hoermann, R (December 2015). "Of rats and men: thyroid homeostasis in rodents and human beings.". The Lancet Diabetes & Endocrinology 3 (12): 932–933. doi:10.1016/S2213-8587(15)00421-0. PMID 26590684.
- ↑ Hoermann, R; Midgley, JE; Larisch, R; Dietrich, JW (2015). "Homeostatic Control of the Thyroid-Pituitary Axis: Perspectives for Diagnosis and Treatment.". Frontiers in Endocrinology 6: 177. doi:10.3389/fendo.2015.00177. PMID 26635726.
- ↑ "Integration of Peripheral and Glandular Regulation of Triiodothyronine Production by Thyrotropin in Untreated and Thyroxine-Treated Subjects". Hormone and Metabolic Research 47 (9): 674–80. 2015. doi:10.1055/s-0034-1398616. PMID 25750078. https://zenodo.org/record/918309.
- ↑ Hoermann, R; Midgley, JEM; Dietrich, JW; Larisch, R (June 2017). "Dual control of pituitary thyroid stimulating hormone secretion by thyroxine and triiodothyronine in athyreotic patients.". Therapeutic Advances in Endocrinology and Metabolism 8 (6): 83–95. doi:10.1177/2042018817716401. PMID 28794850.
- ↑ Fliers, E; Kalsbeek, A; Boelen, A (November 2014). "Beyond the fixed setpoint of the hypothalamus-pituitary-thyroid axis.". European Journal of Endocrinology 171 (5): R197–208. doi:10.1530/EJE-14-0285. PMID 25005935. https://pure.knaw.nl/ws/files/1060474/Fliers2014EurJEndocrinol.pdf.
- ↑ Jump up to: 12.0 12.1 Chatzitomaris, Apostolos; Hoermann, Rudolf; Midgley, John E.; Hering, Steffen; Urban, Aline; Dietrich, Barbara; Abood, Assjana; Klein, Harald H. et al. (20 July 2017). "Thyroid Allostasis–Adaptive Responses of Thyrotropic Feedback Control to Conditions of Strain, Stress, and Developmental Programming". Frontiers in Endocrinology 8: 163. doi:10.3389/fendo.2017.00163. PMID 28775711.
- ↑ "Thyrotropin receptor autoantibodies are associated with continued thyrotropin suppression in treated euthyroid Graves' disease patients". Journal of Clinical Endocrinology & Metabolism 88 (9): 4135–4138. 2003. doi:10.1210/jc.2003-030430. PMID 12970276.
- ↑ "Complex relationship between free thyroxine and TSH in the regulation of thyroid function". European Journal of Endocrinology 162 (6): 1123–9. 2010. doi:10.1530/EJE-10-0106. PMID 20299491.
- ↑ "The relationship between serum TSH and free T4 in older people". Journal of Clinical Pathology 65 (5): 463–5. 2012. doi:10.1136/jclinpath-2011-200433. PMID 22287691.
- ↑ "Is pituitary TSH an adequate measure of thyroid hormone-controlled homoeostasis during thyroxine treatment?". European Journal of Endocrinology 168 (2): 271–80. 2012. doi:10.1530/EJE-12-0819. PMID 23184912.
- ↑ "Physiological states and functional relation between thyrotropin and free thyroxine in thyroid health and disease: In vivo and in silico data suggest a hierarchical model". Journal of Clinical Pathology 66 (4): 335–42. 2013. doi:10.1136/jclinpath-2012-201213. PMID 23423518.
- ↑ "Regulation of the pituitary-thyroid axis in man: Relationship of TSH concentration to concentration of free and total thyroxine in plasma". The Journal of Clinical Endocrinology and Metabolism 27 (2): 251–5. 1967. doi:10.1210/jcem-27-2-251. PMID 4163614.
- ↑ "Ghrelin affects the hypothalamus–pituitary–thyroid axis in humans by increasing free thyroxine and decreasing TSH in plasma". European Journal of Endocrinology 162 (6): 1059–1065. 2010. doi:10.1530/EJE-10-0094. PMID 20423986.
- ↑ "Nonthyroidal illness syndrome: Is it far away from Crohn's disease?". Journal of Clinical Gastroenterology 47 (2): 153–9. 2013. doi:10.1097/MCG.0b013e318254ea8a. PMID 22874844.
- ↑ Jump up to: 21.0 21.1 Dietrich, J. W. (2002). Der Hypophysen-Schilddrüsen-Regelkreis. Berlin, Germany: Logos-Verlag Berlin. 3897228505. ISBN 978-3-89722-850-4. OCLC 50451543.
- ↑ "The use of thyroid function tests in the diagnosis of hypopituitarism: Definition and evaluation of the TSH Index". Clinical Endocrinology 71 (4): 529–34. 2009. doi:10.1111/j.1365-2265.2009.03534.x. PMID 19226261.
Further reading
- Dietrich J W, Tesche A, Pickardt C R, Mitzdorf U (2004). "Thyrotropic Feedback Control: Evidence for an Additional Ultrashort Feedback Loop from Fractal Analysis". Cybernetics and Systems 35 (4): 315–331. doi:10.1080/01969720490443354.
- Gauna C, van den Berghe G H, van der Lely A J (2005). "Pituitary Function During Severe and Life-threatening Illnesses". Pituitary 8 (3–4): 213–217. doi:10.1007/s11102-006-6043-3. PMID 16508715.
- Dietrich, Johannes W.; Midgley, John E. M.; Hoermann, Rudolf (2018). Homeostasis and allostasis of thyroid function. Lausanne: Frontiers Media SA. ISBN 9782889455706.
 |

