Biology:Intelectin
| Xenopus embryonic epidermal lectin | |||||||
|---|---|---|---|---|---|---|---|
| Error creating thumbnail: Unable to save thumbnail to destination Monomeric structure of XEEL-CRD with bound D-glycerol 1-phosphate. The protein is colored using a blue-red gradient from the N- to the C- terminus. Calcium ions are shown as green spheres and the coordinated water molecules are shown as red spheres. | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | itln1 | ||||||
| Entrez | 398574 | ||||||
| HomoloGene | 111044 | ||||||
| PDB | 4WN0 (ECOD) | ||||||
| RefSeq (mRNA) | NM_001089101.1 | ||||||
| RefSeq (Prot) | NP_001082570.1 | ||||||
| UniProt | Q800K0 | ||||||
| |||||||
| Human intelectin-1 | |
|---|---|
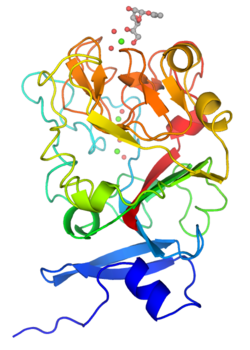 Monomeric structure of human intelectin with bound allyl-beta-D-galactofuranose. The protein is colored using a blue-red gradient from the N- to the C- terminus. Calcium ions are shown as green spheres and the coordinated water molecules are shown as red spheres. | |
| Identifiers | |
| Symbol | ITLN1 |
| Alt. symbols | hIntL-1 |
| NCBI gene | 55600 |
| HGNC | 18259 |
| OMIM | 609873 |
| PDB | 4WMY |
| RefSeq | NP_060095 |
| UniProt | Q8WWA0 |
| Other data | |
| Locus | Chr. 1 q21.3 |
Intelectins are lectins (carbohydrate-binding proteins) expressed in humans and other chordates. Humans express two types of intelectins encoded by ITLN1 and ITLN2 genes respectively.[1][2] Several intelectins bind microbe-specific carbohydrate residues. Therefore, intelectins have been proposed to function as immune lectins.[3][4] Even though intelectins contain fibrinogen-like domain found in the ficolins family of immune lectins, there is significant structural divergence.[5] Thus, intelectins may not function through the same lectin-complement pathway. Most intelectins are still poorly characterized and they may have diverse biological roles. Human intelectin-1 (hIntL-1) has also been shown to bind lactoferrin,[6] but the functional consequence has yet to be elucidated. Additionally, hIntL-1 is a major component of asthmatic mucus[7] and may be involved in insulin physiology as well.[8]
Diversity
The first intelectin was discovered in Xenopus laevis oocyte and is named XL35 or XCGL-1.[9][10][11] X. laevis oocyte also contains a closely related XCGL-2.[12] In addition, X. laevis embryos secrete Xenopus embryonic epidermal lectin into the environmental water, presumably to bind microbes.[13][14] XSL-1 and XSL-2 are also expressed in X. laevis serum when stimulated with lipopolysaccharide.[15] Two additional intestinal intelectins are discovered in X. laevis[16]
Human has two intelectins: hIntL-1 (omentin) and hIntL-2.[17] Mouse also has two intelectins: mIntL-1 and mIntL-2.[18]
Immune system
Several lines of evidence suggest that intelectins recognize microbes and may function as an innate immune defense protein. Tunicate intelectin is an opsonin for phagocytosis by hemocyte.[19] Amphioxus intelectin has been shown to agglutinate bacteria.[20][21] In zebrafish and rainbow trout, intelectin expression is stimulated upon microbial exposure.[22][23][24] Mammals such as sheep and mice also upregulate intelectin expression upon parasitic infection.[25][26] Increase in intelectin expression upon microbial exposure support the hypothesis that intelectins play a role in the immune system.
Structure
Although intelectins require calcium ion for function, the sequences bear no resemblance to C-type lectins.[3] In addition, merely around 50 amino acids (the fibronogen-like domain) align with any known protein, specifically the ficolin family.[2] The first structural details of an intelectin comes from the crystal structure of selenomethionine-labeled XEEL carbohydrate-recognition domain (Se-Met XEEL-CRD) solved by Se-SAD.[5] XEEL-CRD was expressed and Se-Met-labeled in High Five insect cells using a recombinant baculovirus. The fibrinogen-like fold is conserved despite amino acid sequence divergence. However, extensive insertions are present in intelectin compared to ficolins, thus making intelectin a distinct lectin structural class.[5] The Se-Met XEEL-CRD structure then enables the structure solution by molecular replacement of D-glycerol 1-phosphate (GroP)-bound XEEL-CRD,[5] apo-human intelectin-1 (hIntL-1),[4] and galactofuranose-bound hIntL-1.[4]
Each polypeptide chain of XEEL and hIntL-1 contains three bound calcium ions: two in the structural calcium site and one in the ligand binding site.[4][5] The amino acid residues in the structural calcium site are conserved among intelectins, thus it is likely that most, if not all, intelectins have two structural calcium ions.[5]
In the ligand binding site of XEEL and hIntL-1, the exocyclic vicinal diol of the carbohydrate ligand directly coordinates to the calcium ion.[4][5] There are large variations in the ligand binding site residues among intelectin homologs suggesting that the intelectin family may have broad ligand specificities and biological functions.[5] As there is no intelectin numbering conventions in different organisms, one should not assume functional homology based on the intelectin number. For example, hIntL-1 has glutamic acid residues in the ligand binding site to coordinate a calcium ion, while zebrafish intelectin-1 are devoided of these acidic residues.[5] Zebrafish intelectin-2 ligand binding site residues are similar to those present in hIntL-1.
-
Xenopus embryonic epidermal lectin (XEEL) ligand binding site with bound D-glycerol 1-phosphate. The calcium ion is shown as a green sphere and the ordered water molecules are shown as red spheres.[5]
-
Human intelectin-1 (hIntL-1) ligand binding site with bound allyl-beta-D-galactofuranose. The calcium ion is shown as a green sphere and the ordered water molecules are shown as red spheres.[4]
Oligomeric state
hIntL-1 is a disulfide-linked trimer as shown by non-reducing SDS-PAGE[3] and X-ray crystallography.[4] Despite lacking the intermolecular disulfide bonds, XEEL-CRD is trimeric in solution.[5] The N-terminal peptide of the full length XEEL is responsible for dimerizing the trimeric XEEL-CRD into a disulfide-linked hexameric full-length XEEL.[5] Therefore, the N-termini of intelectins are often responsible for forming disulfide-linked oligomer. In intelectin homologs where the N-terminal cysteines are absent, the CRD itself may still capable of forming non-covalent oligomer in solution.
-
Disulfide-linked trimeric human intelectin-1.[4]
-
Trimeric Xenopus embryonic epidermal lectin carbohydrate-recognition domain (XEEL-CRD). Extensive biophysical investigations conclusively indicate that XEEL-CRD is trimeric in solution despite lacking the intermolecular disulfide bonds found in hIntL-1.[5]
References
- ↑ "The X-lectins: a new family with homology to the Xenopus laevis oocyte lectin XL-35". Glycoconjugate Journal 21 (8–9): 443–50. August 2004. doi:10.1007/s10719-004-5534-6. PMID 15750785.
- ↑ 2.0 2.1 "Comparative genomic and phylogenetic analyses of the intelectin gene family: implications for their origin and evolution". Developmental and Comparative Immunology 41 (2): 189–99. Oct 2013. doi:10.1016/j.dci.2013.04.016. PMID 23643964.
- ↑ 3.0 3.1 3.2 "Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall". The Journal of Biological Chemistry 276 (26): 23456–63. Jun 2001. doi:10.1074/jbc.M103162200. PMID 11313366.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 "Recognition of microbial glycans by human intelectin-1". Nature Structural & Molecular Biology 22 (8): 603–10. Aug 2015. doi:10.1038/nsmb.3053. PMID 26148048.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 "Structures of Xenopus embryonic epidermal lectin reveal a conserved mechanism of microbial glycan recognition". The Journal of Biological Chemistry 291 (11): 5596–610. Jan 2016. doi:10.1074/jbc.M115.709212. PMID 26755729.
- ↑ "Molecular cloning and functional expression of a human intestinal lactoferrin receptor". Biochemistry 40 (51): 15771–9. Dec 2001. doi:10.1021/bi0155899. PMID 11747454.
- ↑ "Intelectin-1 is a prominent protein constituent of pathologic mucus associated with eosinophilic airway inflammation in asthma". American Journal of Respiratory and Critical Care Medicine 189 (8): 1005–7. Apr 2014. doi:10.1164/rccm.201312-2220LE. PMID 24735037.
- ↑ "Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action". American Journal of Physiology. Endocrinology and Metabolism 290 (6): E1253–61. Jun 2006. doi:10.1152/ajpendo.00572.2004. PMID 16531507.
- ↑ "Lectin from embryos and oocytes of Xenopus laevis. Purification and properties". The Journal of Biological Chemistry 257 (13): 7520–4. Jul 1982. doi:10.1016/S0021-9258(18)34409-0. PMID 7085636.
- ↑ "Isolation and characterization of a lectin from the cortical granules of Xenopus laevis eggs". Biochemistry 25 (20): 6013–20. Oct 1986. doi:10.1021/bi00368a027. PMID 3098282.
- ↑ "Cloning and expression of a Xenopus laevis oocyte lectin and characterization of its mRNA levels during early development". Glycobiology 7 (3): 367–72. Apr 1997. doi:10.1093/glycob/7.3.367. PMID 9147045.
- ↑ "Xenopus galectin-VIIa binds N-glycans of members of the cortical granule lectin family (xCGL and xCGL2)". Glycobiology 15 (7): 709–20. Jul 2005. doi:10.1093/glycob/cwi051. PMID 15761024.
- ↑ "Developmental expression of XEEL, a novel molecule of the Xenopus oocyte cortical granule lectin family". Development Genes and Evolution 213 (7): 368–70. Jul 2003. doi:10.1007/s00427-003-0341-9. PMID 12802587.
- ↑ "Isolation, characterization, and extra-embryonic secretion of the Xenopus laevis embryonic epidermal lectin, XEEL". Glycobiology 15 (3): 281–90. Mar 2005. doi:10.1093/glycob/cwi010. PMID 15537792.
- ↑ "Bacterial lipopolysaccharides stimulate production of XCL1, a calcium-dependent lipopolysaccharide-binding serum lectin, in Xenopus laevis". Developmental and Comparative Immunology 40 (2): 94–102. Jun 2013. doi:10.1016/j.dci.2013.02.008. PMID 23454582.
- ↑ "Identification and characterization of a novel intelectin in the digestive tract of Xenopus laevis". Developmental and Comparative Immunology 59: 229–239. Feb 2016. doi:10.1016/j.dci.2016.02.006. PMID 26855011.
- ↑ "Human homologs of the Xenopus oocyte cortical granule lectin XL35". Glycobiology 11 (1): 65–73. Jan 2001. doi:10.1093/glycob/11.1.65. PMID 11181563.
- ↑ "Strain-specific copy number variation in the intelectin locus on the 129 mouse chromosome 1". BMC Genomics 12 (1): 110. 2011. doi:10.1186/1471-2164-12-110. PMID 21324158.
- ↑ "A unique primary structure, cDNA cloning and function of a galactose-specific lectin from ascidian plasma". European Journal of Biochemistry 261 (1): 33–9. Apr 1999. doi:10.1046/j.1432-1327.1999.00238.x. PMID 10103030.
- ↑ "Identification of an amphioxus intelectin homolog that preferably agglutinates gram-positive over gram-negative bacteria likely due to different binding capacity to LPS and PGN". Fish & Shellfish Immunology 33 (1): 11–20. Jul 2012. doi:10.1016/j.fsi.2012.03.023. PMID 22475783.
- ↑ "Characterization and comparative analyses of two amphioxus intelectins involved in the innate immune response". Fish & Shellfish Immunology 34 (5): 1139–46. May 2013. doi:10.1016/j.fsi.2013.01.017. PMID 23428515.
- ↑ "Characterization and comparative analyses of zebrafish intelectins: highly conserved sequences, diversified structures and functions". Fish & Shellfish Immunology 26 (3): 396–405. Mar 2009. doi:10.1016/j.fsi.2008.11.019. PMID 19100836.
- ↑ "Identification, cloning and tissue localization of a rainbow trout (Oncorhynchus mykiss) intelectin-like protein that binds bacteria and chitin". Fish & Shellfish Immunology 25 (1–2): 91–105. Jul 2008. doi:10.1016/j.fsi.2008.02.018. PMID 18502147.
- ↑ "Immunohistochemical localization of rainbow trout ladderlectin and intelectin in healthy and infected rainbow trout (Oncorhynchus mykiss)". Fish & Shellfish Immunology 26 (1): 154–63. Jan 2009. doi:10.1016/j.fsi.2008.03.001. PMID 19046637.
- ↑ "Identification of novel genes in intestinal tissue that are regulated after infection with an intestinal nematode parasite". Infection and Immunity 73 (7): 4025–33. Jul 2005. doi:10.1128/IAI.73.7.4025-4033.2005. PMID 15972490.
- ↑ "Up-regulation of intelectin in sheep after infection with Teladorsagia circumcincta". International Journal for Parasitology 38 (3–4): 467–75. Mar 2008. doi:10.1016/j.ijpara.2007.08.015. PMID 17983620.
Further reading
- "Recognition of microbial glycans by human intelectin-1". Nature Structural & Molecular Biology 22 (8): 603–10. Aug 2015. doi:10.1038/nsmb.3053. PMID 26148048. for exhaustive ligand binding analysis of human intelectin-1 (hIntL-1). The article also reveals how hIntL-1 could discriminate between microbial and mammalian cells.
- "Structures of Xenopus embryonic epidermal lectin reveal a conserved mechanism of microbial glycan recognition". The Journal of Biological Chemistry 291 (11): 5596–610. Jan 2016. doi:10.1074/jbc.M115.709212. PMID 26755729. for discussion on how the first intelectin structure (XEEL-CRD) was solved. In depth biophysical and evolutionary analyses of the intelectin family in the light of the available 3D structures also provide significant insights into this protein family not previously appreciated. The article serves as the most up-to-date review on the biochemistry of the intelectin family.
- "Comparative genomic and phylogenetic analyses of the intelectin gene family: implications for their origin and evolution". Developmental and Comparative Immunology 41 (2): 189–99. Oct 2013. doi:10.1016/j.dci.2013.04.016. PMID 23643964. for comprehensive genomics analysis of intelectins from various organisms.
 |
![Xenopus embryonic epidermal lectin (XEEL) ligand binding site with bound D-glycerol 1-phosphate. The calcium ion is shown as a green sphere and the ordered water molecules are shown as red spheres.[5]](/wiki/images/thumb/f/fc/XEEL_ligand_binding_site.jpg/300px-XEEL_ligand_binding_site.jpg)
![Human intelectin-1 (hIntL-1) ligand binding site with bound allyl-beta-D-galactofuranose. The calcium ion is shown as a green sphere and the ordered water molecules are shown as red spheres.[4]](/wiki/images/thumb/8/8a/Human_intelectin-1_ligand_binding_site.jpg/300px-Human_intelectin-1_ligand_binding_site.jpg)
![Disulfide-linked trimeric human intelectin-1.[4]](/wiki/images/thumb/7/76/Trimeric_human_intelectin-1.png/257px-Trimeric_human_intelectin-1.png)
![Trimeric Xenopus embryonic epidermal lectin carbohydrate-recognition domain (XEEL-CRD). Extensive biophysical investigations conclusively indicate that XEEL-CRD is trimeric in solution despite lacking the intermolecular disulfide bonds found in hIntL-1.[5]](/wiki/images/thumb/9/95/Trimeric_Xenopus_embryonic_epidermal_lectin_CRD.png/257px-Trimeric_Xenopus_embryonic_epidermal_lectin_CRD.png)
