Biology:MBD4
 Generic protein structure example |
Methyl-CpG-binding domain protein 4 is a protein that in humans is encoded by the MBD4 gene.[1][2][3]
Structure
Human MBD4 protein has 580 amino acids with a methyl-CpG-binding domain at amino acids 82–147 and a C-terminal DNA glycosylase domain at amino acids 426–580.[4] These domains are separated by an intervening region that interacts with UHRF1, an E3 ubiquitin ligase, and USP7, a de-ubiquinating enzyme.[5]
Function
DNA methylation is the major modification of eukaryotic genomes and plays an essential role in mammalian development. Human proteins MECP2, MBD1, MBD2, MBD3, and MBD4 (this gene) comprise a family of nuclear proteins related by the presence in each of a methyl-CpG-binding domain (MBD). Each of these proteins, with the exception of MBD3, is capable of binding specifically to methylated DNA. MBD4 may function to mediate the biological consequences of the methylation signal. In addition, MBD4 has protein sequence similarity to bacterial DNA repair enzymes and thus may have some function in DNA repair. Further, MBD4 gene mutations are detected in tumors with primary microsatellite instability (MSI), a form of genomic instability associated with defective DNA mismatch repair, and MBD4 gene meets 4 of 5 criteria of a bona fide MIS target gene.[3]
Deaminated bases as targets
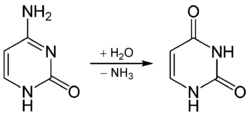
Bases in DNA decay spontaneously, and this decay includes hydrolytic deamination of purines and pyrimidines that contain an exocyclic amino group (see image). Hypoxanthine and xanthine are generated at a relatively slow rate by deamination of adenine and guanine, respectively. However, deamination of pyrimidines occurs at a 50-fold higher rate of approximately 200–300 events per cell per day,[4] and is potentially highly mutagenic. Deamination of cytosine (C) to uracil (U) and 5-methylcytosine (5mC) to thymine (T) generates G:U and G:T mismatches, respectively. Upon DNA replication, these mismatches cause C to T transition mutations. Notably, for 5mC deamination, these mutations arise predominantly in the context of CpG sites. The deamination rate of 5mC is approximately three times that of C. MBD4 protein binds preferentially to fully methylated CpG sites and to their deamination derivatives G:U and G:T base pairs.[4] MBD4, which is employed in an initial step of base excision repair, specifically catalyzes the removal of T and U paired with guanine (G) within CpG sites.[6]
Mutational importance of targets
G:U and G:T mismatches, upon DNA replication, give rise to C to T transition mutations.[6] The mismatched U or T is usually removed by MBD4 before replication, thus avoiding mutation. Alternatively, for G:T mismatches, the T may be removed by thymine-DNA glycosylase. Mutations in the MBD4 gene (especially expansions/deletions in the polyadenine regions of the MBD4 gene) increase the genomic instability phenotype of a subset of MMR-defective tumors in mice, specifically contributing to elevated G:C to A:T transitions.[7]
About 1/3 of all intragenic single base pair mutations in human cancers occur in CpG dinucleotides and are the result of C to T or G to A transitions.[6][8] These transitions comprise the most frequent mutations in human cancer. For example, nearly 50% of somatic mutations of the tumor suppressor gene p53 in colorectal cancer are G:C to A:T transitions within CpG sites.[6]
Clinical significance in cancer
Germline mutations of MBD4
Germline mutations of MBD4 have been identified in acute myeloid leukemias, uveal melanomas, and glioblastomas.[9][10][11] These cases presented an inactivation of the second allele of MBD4 in tumor and were associated with a subsequent very high mutation burden at CpG dinucleotides.
Somatic mutations of MBD4
Mutation of MBD4 occurs in about 4% of colorectal cancers.[7] MBD4 mutations also occur in tumor samples of melanoma, ovarian, lung, esophageal and prostate cancers at frequencies between 0.5% and 8%.[7]
MBD4 has a special relationship with DNA mismatch repair (MMR). MBD4 protein binds strongly to the MMR protein MLH1.[2] A mutational deficiency in MBD4 causes down-regulation, at the protein level, of MMR proteins Mlh1, Msh2, Pms2, and Msh6 by 5.8-, 5.6-, 2.6-, and 2.7-fold, respectively.[12] In colorectal cancers with mutations in MMR genes, co-occurrence of MBD4 mutations were found in 27% of cancers.[7]
Epigenetic silencing
MBD4 mRNA expression is reduced in colorectal neoplasms due to methylation of the promoter region of MBD4.[13] A majority of histologically normal fields surrounding the neoplastic growths also show reduced MBD4 mRNA expression (a field defect) compared to histologically normal tissue from individuals who never had a colonic neoplasm. This indicates that an epigenetic deficiency in MBD4 expression is a frequent early event in colorectal tumorigenesis.
While other DNA repair genes, such as MGMT and MLH1, are often evaluated for epigenetic repression in many types of cancer,[citation needed] epigenetic deficiency of MBD4 is usually not evaluated, but might be of importance in such cancers as well.
Response to checkpoint inhibitors
MBD4-associated hypermutated profile was shown to be associated with a tumor regression when a uveal melanoma patient was treated with a checkpoint inhibitor making these mutations potential biomarkers to treat cancers.[11]
Interactions
MBD4 has been shown to interact with MLH1[2] and FADD.[14]
References
- ↑ "Identification and Characterization of a Family of Mammalian Methyl-CpG Binding Proteins". Mol Cell Biol 18 (11): 6538–47. Nov 1998. doi:10.1128/mcb.18.11.6538. PMID 9774669.
- ↑ 2.0 2.1 2.2 "MED1, a novel human methyl-CpG-binding endonuclease, interacts with DNA mismatch repair protein MLH1". Proc Natl Acad Sci U S A 96 (7): 3969–74. May 1999. doi:10.1073/pnas.96.7.3969. PMID 10097147. Bibcode: 1999PNAS...96.3969B.
- ↑ 3.0 3.1 "Entrez Gene: MBD4 methyl-CpG binding domain protein 4". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=8930.
- ↑ 4.0 4.1 4.2 "Role of base excision repair in maintaining the genetic and epigenetic integrity of CpG sites". DNA Repair 32: 33–42. Aug 2015. doi:10.1016/j.dnarep.2015.04.011. PMID 26021671.
- ↑ "MBD4 interacts with and recruits USP7 to heterochromatic foci". Journal of Cellular Biochemistry 116 (3): 476–85. Mar 2015. doi:10.1002/jcb.25001. PMID 25358258.
- ↑ 6.0 6.1 6.2 6.3 "MBD4 and TDG: multifaceted DNA glycosylases with ever expanding biological roles". Mutation Research 743–744: 12–25. 2013. doi:10.1016/j.mrfmmm.2012.11.001. PMID 23195996.
- ↑ 7.0 7.1 7.2 7.3 "Involvement of MBD4 inactivation in mismatch repair-deficient tumorigenesis". Oncotarget 6 (40): 42892–904. Oct 2015. doi:10.18632/oncotarget.5740. PMID 26503472. PMC 4767479. https://dash.harvard.edu/bitstream/handle/1/26318553/4767479.pdf?sequence=1.
- ↑ "The CpG dinucleotide and human genetic disease". Human Genetics 78 (2): 151–5. Feb 1988. doi:10.1007/bf00278187. PMID 3338800.
- ↑ "MBD4 guards against methylation damage and germline deficiency predisposes to clonal hematopoiesis and early-onset AML". Blood 132 (14): 1526–1534. Jul 2018. doi:10.1182/blood-2018-05-852566. PMID 30049810.
- ↑ Waszak SM, Tiao G, Zhu B, Rausch T, et al. (Nov 2017). "Germline determinants of the somatic mutation landscape in 2,642 cancer genomes". bioRxiv 10.1101/208330.
- ↑ 11.0 11.1 "Outlier response to anti-PD1 in uveal melanoma reveals germline MBD4 mutations in hypermutated tumors.". Nature Communications 9 (1): 1866. May 2018. doi:10.1038/s41467-018-04322-5. PMID 29760383. Bibcode: 2018NatCo...9.1866R.
- ↑ "The base excision repair enzyme MED1 mediates DNA damage response to antitumor drugs and is associated with mismatch repair system integrity". Proceedings of the National Academy of Sciences of the United States of America 100 (25): 15071–6. Dec 2003. doi:10.1073/pnas.2334585100. PMID 14614141.
- ↑ "Epigenetic downregulation of the DNA repair gene MED1/MBD4 in colorectal and ovarian cancer". Cancer Biology & Therapy 8 (1): 94–100. Jan 2009. doi:10.4161/cbt.8.1.7469. PMID 19127118.
- ↑ "Fas-associated death domain protein interacts with methyl-CpG binding domain protein 4: A potential link between genome surveillance and apoptosis". Proc. Natl. Acad. Sci. U.S.A. 100 (9): 5211–6. Apr 2003. doi:10.1073/pnas.0431215100. PMID 12702765. Bibcode: 2003PNAS..100.5211S.
Further reading
- "A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer". Cancer Res. 58 (22): 5248–57. 1998. PMID 9823339.
- "Genomic structure and chromosomal mapping of the murine and human Mbd1, Mbd2, Mbd3, and Mbd4 genes". Mamm. Genome 10 (9): 906–12. 1999. doi:10.1007/s003359901112. PMID 10441743.
- "The thymine glycosylase MBD4 can bind to the product of deamination at methylated CpG sites". Nature 401 (6750): 301–4. 1999. doi:10.1038/45843. PMID 10499592. Bibcode: 1999Natur.401..301H.
- "The DNA repair gene MBD4 (MED1) is mutated in human carcinomas with microsatellite instability". Nat. Genet. 23 (3): 266–8. 1999. doi:10.1038/15443. PMID 10545939.
- "Biphasic kinetics of the human DNA repair protein MED1 (MBD4), a mismatch-specific DNA N-glycosylase". J. Biol. Chem. 275 (42): 32422–9. 2000. doi:10.1074/jbc.M004535200. PMID 10930409.
- "Investigation of the substrate spectrum of the human mismatch-specific DNA N-glycosylase MED1 (MBD4): fundamental role of the catalytic domain". J. Cell. Physiol. 185 (3): 473–80. 2000. doi:10.1002/1097-4652(200012)185:3<473::AID-JCP19>3.0.CO;2-#. PMID 11056019.
- "Expression of the genes of methyl-binding domain proteins in human gliomas". Oncol. Rep. 9 (2): 393–5. 2002. doi:10.3892/or.9.2.393. PMID 11836615.
- "Estradiol receptor potentiates, in vitro, the activity of 5-methylcytosine DNA glycosylase". FEBS Lett. 527 (1–3): 63–6. 2002. doi:10.1016/S0014-5793(02)03166-6. PMID 12220634.
- "Frameshift mutations in the MBD4/MED1 gene in primary gastric cancer with high-frequency microsatellite instability". Cancer Lett. 181 (1): 115–20. 2002. doi:10.1016/S0304-3835(02)00043-5. PMID 12430186.
- "Fas-associated death domain protein interacts with methyl-CpG binding domain protein 4: A potential link between genome surveillance and apoptosis". Proc. Natl. Acad. Sci. U.S.A. 100 (9): 5211–6. 2003. doi:10.1073/pnas.0431215100. PMID 12702765. Bibcode: 2003PNAS..100.5211S.
- "Microsatellite instability and MBD4 mutation in unselected colorectal cancer". Anticancer Res. 23 (4): 3569–74. 2003. PMID 12926109.
- "Analysis of a high-throughput yeast two-hybrid system and its use to predict the function of intracellular proteins encoded within the human MHC class III region". Genomics 83 (1): 153–67. 2004. doi:10.1016/S0888-7543(03)00235-0. PMID 14667819.
- "The Thymine DNA Glycosylase MBD4 Represses Transcription and Is Associated with Methylated p16INK4a and hMLH1 Genes". Mol. Cell. Biol. 25 (11): 4388–96. 2005. doi:10.1128/MCB.25.11.4388-4396.2005. PMID 15899845.
- "MED1/TRAP220 exists predominantly in a TRAP/ Mediator subpopulation enriched in RNA polymerase II and is required for ER-mediated transcription". Mol. Cell 19 (1): 89–100. 2005. doi:10.1016/j.molcel.2005.05.015. PMID 15989967.