Medicine:Glioblastoma
| Glioblastoma | |
|---|---|
| Other names | Glioblastoma multiforme |
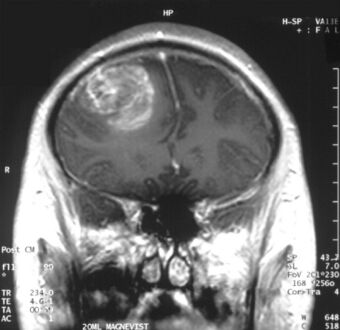 | |
| Coronal MRI with contrast of a glioblastoma in a 15-year-old male | |
| Specialty | Neuro-oncology, neurosurgery |
| Symptoms | Initially nonspecific, headaches, personality changes, nausea, symptoms similar to a stroke[1] |
| Usual onset | ~ 64 years old[2][3] |
| Causes | Usually unclear[2] |
| Risk factors | Genetic disorders (neurofibromatosis, Li–Fraumeni syndrome), previous radiation therapy[2][3] |
| Diagnostic method | CT scan, MRI scan, tissue biopsy[1] |
| Prevention | Unknown[3] |
| Treatment | Surgery, chemotherapy, radiation[3] |
| Medication | Temozolomide, steroids[1][4] |
| Prognosis | Life expectancy ~ 12 months with treatment (5 year survival <10%)[2][5] |
| Frequency | 3 per 100,000 per year[3] |
Glioblastoma, previously known as glioblastoma multiforme (GBM), is the most aggressive and most common type of cancer that originates in the brain, and has very poor prognosis for survival.[6][7][8] Initial signs and symptoms of glioblastoma are nonspecific.[1] They may include headaches, personality changes, nausea, and symptoms similar to those of a stroke.[1] Symptoms often worsen rapidly and may progress to unconsciousness.[2]
The cause of most cases of glioblastoma is not known.[2] Uncommon risk factors include genetic disorders, such as neurofibromatosis and Li–Fraumeni syndrome, and previous radiation therapy.[2][3] Glioblastomas represent 15% of all brain tumors.[1] They are thought to arise from astrocytes.[9] The diagnosis typically is made by a combination of a CT scan, MRI scan, and tissue biopsy.[1]
There is no known method of preventing the cancer.[3] Treatment usually involves surgery, after which chemotherapy and radiation therapy are used.[3] The medication temozolomide is frequently used as part of chemotherapy.[3][4][10] High-dose steroids may be used to help reduce swelling and decrease symptoms.[1] Surgical removal (decompression) of the tumor is linked to increased survival, but only by some months.[11]
Despite maximum treatment, the cancer almost always recurs.[3] The typical duration of survival following diagnosis is 10–13 months, with fewer than 5–10% of people surviving longer than five years.[12][13][5] Without treatment, survival is typically three months.[14] It is the most common cancer that begins within the brain and the second-most common brain tumor, after meningioma, which is benign in most cases.[6][15] About 3 in 100,000 people develop the disease per year.[3] The average age at diagnosis is 64, and the disease occurs more commonly in males than females.[2][3]
Tumors of the central nervous system are the 10th leading cause of death worldwide, with up to 90% being brain tumors.[16] Glioblastoma multiforme (GBM) is derived from astrocytes and accounts for 49% of all malignant central nervous system tumors, making it the most common form of central nervous system cancer. Despite countless efforts to develop new therapies for GBM over the years, the median survival rate of GBM patients worldwide is a dismal 8 months, with radiation and chemotherapy standard-of-care treatment beginning shortly after diagnosis only improving median survival length to around 14 months and a five-year survival rate of 5-10%. Similarly, the five-year survival rate for individuals with any form of primary malignant brain tumor is only 20%.[17] The challenges associated with successfully treating brain cancers are numerous. In the simplest terms, brain tumors often occur in areas too difficult or dangerous to surgically resect, and most drug therapeutics are incapable of crossing the blood-brain barrier in sufficient quantities to stop tumor growth. Furthermore, while stereotactic radiosurgery-based approaches have proved to be effective for ablating a variety of brain tumors visible to MRI and other neuroimaging, metastatic brain cancers maintain high recurrence rates, with GBM recurrence seemingly inevitable.
Signs and symptoms
Common symptoms include seizures, headaches, nausea and vomiting, memory loss, changes to personality, mood or concentration, and localized neurological problems.[18] The kinds of symptoms produced depend more on the location of the tumor than on its pathological properties. The tumor can start producing symptoms quickly, but occasionally is an asymptomatic condition until it reaches an enormous size.[19]
Risk factors
The cause of most cases is unclear.[2] The best known risk factor is exposure to ionizing radiation, and CT scan radiation is an important cause.[20][21] About 5% develop from certain hereditary syndromes.[18]
Genetics
Uncommon risk factors include genetic disorders such as neurofibromatosis, Li–Fraumeni syndrome, tuberous sclerosis, or Turcot syndrome.[18] Previous radiation therapy is also a risk.[2][3] For unknown reasons, it occurs more commonly in males.[22]
Environmental
Other associations include exposure to smoking, pesticides, and working in petroleum refining or rubber manufacturing.[18]
Glioblastoma has been associated with the viruses SV40,[23] HHV-6,[24][25] and cytomegalovirus.[26]
Other
Research has been done to see if consumption of cured meat is a risk factor. No risk had been confirmed as of 2003.[27] Similarly, exposure to formaldehyde, and residential electromagnetic fields, such as from cell phones and electrical wiring within homes, have been studied as risk factors. As of 2015, they had not been shown to cause GBM.[18][28][29]
Pathogenesis
The cellular origin of glioblastoma is unknown. Because of the similarities in immunostaining of glial cells and glioblastoma, gliomas such as glioblastoma have long been assumed to originate from glial-type stem cells found in the subventricular zone. More recent studies suggest that astrocytes, oligodendrocyte progenitor cells, and neural stem cells could all serve as the cell of origin.[30][31]
GBMs usually form in the cerebral white matter, grow quickly, and can become very large before producing symptoms. The tumor may extend into the meninges or ventricular wall, leading to high protein content in the cerebrospinal fluid (CSF) (> 100 mg/dl), as well as an occasional pleocytosis of 10 to 100 cells, mostly lymphocytes. Malignant cells carried in the CSF may spread (rarely) to the spinal cord or cause meningeal gliomatosis. However, metastasis of GBM beyond the central nervous system is extremely unusual. About 50% of GBMs occupy more than one lobe of a hemisphere or are bilateral. Tumors of this type usually arise from the cerebrum and may exhibit the classic infiltration across the corpus callosum, producing a butterfly (bilateral) glioma.[32]
Glioblastoma classification
Brain tumor classification has been traditionally based on histopathology at macroscopic level, measured in hematoxylin-eosin sections. The World Health Organization published the first standard classification in 1979[33] and has been doing so since. The 2007 WHO Classification of Tumors of the Central Nervous System[34] was the last classification mainly based on microscopy features. The new 2016 WHO Classification of Tumors of the Central Nervous System[35] was a paradigm shift: some of the tumors were defined also by their genetic composition as well as their cell morphology.
In 2021, the fifth edition of the WHO Classification of Tumors of the Central Nervous System was released. This update eliminated the classification of secondary glioblastoma and reclassified those tumors as Astrocytoma, IDH mutant, grade 4. Only tumors that are IDH wild type are now classified as glioblastoma.[36]
| Synonyms | Glioblastoma, GBM |
| Cell of origin | Astrocyte |
| Median age at diagnosis | ~62 years |
| Male:Female ratio | 1.42:1 |
| Median length of clinical history at diagnosis | 4 months |
| Median overall survival | |
| Surgery + radiotherapy | 9.9 months |
| Surgery + radiotherapy + chemotherapy | 15 months |
| Location | Usually supratentorial, rarely cerebellum or spine |
| Necrosis and microvascular proliferation | Extensive |
| Associated molecular/genetic mutations | TERT promoter mutation, combined gain of chromosome 7 and loss of chromosome 10; EGFR amplification |
Molecular alterations
There are currently three molecular subtypes of glioblastoma that were identified based on gene expression:[38]
- Classical: Around 97% of tumors in this subtype carry extra copies of the epidermal growth factor receptor (EGFR) gene, and most have higher than normal expression of EGFR, whereas the gene TP53 (p53), which is often mutated in glioblastoma, is rarely mutated in this subtype.[39] Loss of heterozygosity in chromosome 10 is also frequently seen in the classical subtype alongside chromosome 7 amplification.[40]
- The proneural subtype often has high rates of alterations in TP53 (p53), and in PDGFRA the gene encoding a-type platelet-derived growth factor receptor.[41]
- The mesenchymal subtype is characterized by high rates of mutations or other alterations in NF1, the gene encoding neurofibromin 1 and fewer alterations in the EGFR gene and less expression of EGFR than other types.[42]
Initial analyses of gene expression had revealed a fourth neural subtype.[41] However, further analyses revealed that this subtype is non-tumor specific and is potential contamination caused by the normal cells.[38]
Many other genetic alterations have been described in glioblastoma, and the majority of them are clustered in two pathways, the RB and the PI3K/AKT.[43] 68–78% and 88% of Glioblastomas have alterations in these pathways, respectively.[6]
Another important alteration is methylation of MGMT, a "suicide" DNA repair enzyme. Methylation impairs DNA transcription and expression of the MGMT gene. Since the MGMT enzyme can repair only one DNA alkylation due to its suicide repair mechanism, reserve capacity is low and methylation of the MGMT gene promoter greatly affects DNA-repair capacity.[44][45] MGMT methylation is associated with an improved response to treatment with DNA-damaging chemotherapeutics, such as temozolomide.[46]
Studies using genome-wide profiling have revealed glioblastomas to have a remarkable genetic variety.[47]
At least three distinct paths in the development of Glioblastomas have been identified with the aid of molecular investigations.
- The first pathway involves the amplification and mutational activation of receptor tyrosine kinase (RTK) genes, leading to the dysregulation of growth factor signaling. Epithelial growth factor (EGF), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) are all recognized by transmembrane proteins called RTKs. Additionally, they can function as receptors for hormones, cytokines, and other signaling pathways.
- The second method involves activating the intracellular signaling system known as phosphatidylinositol-3-OH kinase (PI3K)/AKT/mTOR, which is crucial for controlling cell survival.
- The third pathway is defined by p53 and retinoblastoma (Rb) tumor suppressor pathway inactivation.[48]
Cancer stem cells
Glioblastoma cells with properties similar to progenitor cells (glioblastoma cancer stem cells) have been found in glioblastomas. Their presence, coupled with the glioblastoma's diffuse nature results in difficulty in removing them completely by surgery, and is therefore believed to be the possible cause behind resistance to conventional treatments, and the high recurrence rate.[49] Glioblastoma cancer stem cells share some resemblance with neural progenitor cells, both expressing the surface receptor CD133.[50] CD44 can also be used as a cancer stem cell marker in a subset of glioblastoma tumour cells.[51] Glioblastoma cancer stem cells appear to exhibit enhanced resistance to radiotherapy and chemotherapy mediated, at least in part, by up-regulation of the DNA damage response.[52]
Metabolism
The IDH1 gene encodes for the enzyme isocitrate dehydrogenase 1 and is not mutated in glioblastoma. As such, these tumors behave more aggressively compared to IDH1-mutated astrocytomas.[45]
Ion channels
Furthermore, GBM exhibits numerous alterations in genes that encode for ion channels, including upregulation of gBK potassium channels and ClC-3 chloride channels. By upregulating these ion channels, glioblastoma tumor cells are hypothesized to facilitate increased ion movement over the cell membrane, thereby increasing H2O movement through osmosis, which aids glioblastoma cells in changing cellular volume very rapidly. This is helpful in their extremely aggressive invasive behavior because quick adaptations in cellular volume can facilitate movement through the sinuous extracellular matrix of the brain.[53]
MicroRNA
As of 2012, RNA interference, usually microRNA, was under investigation in tissue culture, pathology specimens, and preclinical animal models of glioblastoma.[54] Additionally, experimental observations suggest that microRNA-451 is a key regulator of LKB1/AMPK signaling in cultured glioma cells[55] and that miRNA clustering controls epigenetic pathways in the disease.[56]
Tumor vasculature
GBM is characterized by abnormal vessels that present disrupted morphology and functionality.[57] The high permeability and poor perfusion of the vasculature result in a disorganized blood flow within the tumor and can lead to increased hypoxia, which in turn facilitates cancer progression by promoting processes such as immunosuppression.[57][58]
Diagnosis
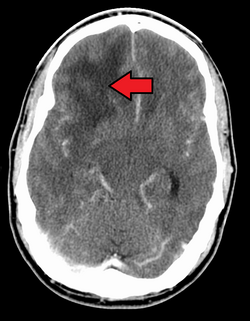

When viewed with MRI, glioblastomas often appear as ring-enhancing lesions. The appearance is not specific, however, as other lesions such as abscess, metastasis, tumefactive multiple sclerosis, and other entities may have a similar appearance.[59] Definitive diagnosis of a suspected GBM on CT or MRI requires a stereotactic biopsy or a craniotomy with tumor resection and pathologic confirmation. Because the tumor grade is based upon the most malignant portion of the tumor, biopsy or subtotal tumor resection can result in undergrading of the lesion. Imaging of tumor blood flow using perfusion MRI and measuring tumor metabolite concentration with MR spectroscopy may add diagnostic value to standard MRI in select cases by showing increased relative cerebral blood volume and increased choline peak, respectively, but pathology remains the gold standard for diagnosis and molecular characterization.[citation needed]
Distinguishing glioblastoma from high-grade astrocytoma is important. These tumors occur spontaneously (de novo) and have not progressed from a lower-grade glioma, as in high-grade astrocytomas[6] Glioblastomas have a worse prognosis and different tumor biology, and may have a different response to therapy, which makes this a critical evaluation to determine patient prognosis and therapy.[44][60] Astrocytomas carry a mutation in IDH1 or IDH2, whereas this mutation is not present in glioblastoma. Thus, IDH1 and IDH2 mutations are a useful tool to distinguish glioblastomas from astrocytomas, since histopathologically they are similar and the distinction without molecular biomarkers is unreliable.[45] IDH-wildtype glioblastomas usually have lower OLIG2 expression compared with IDH-mutant lower grade astrocytomas.[61]
-
Histopathology of glioblastoma, showing high grade astrocytoma features of marked nuclear pleomorphism, multiple mitoses (one at white arrow) and multinucleated cells (one at black arrow), with cells having a patternless arrangement in a pink fibrillary background on H&E stain.
-
Lower magnification histopathology, showing necrosis surrounded by pseudopalisades of tumor cells, conferring a diagnosis of glioblastoma rather than anaplastic astrocytoma
Prevention
There are no known methods to prevent glioblastoma.[3] It is the case for most gliomas, unlike for some other forms of cancer, that they happen without previous warning and there are no known ways to prevent them.[62]
Treatment
Treating glioblastoma is difficult due to several complicating factors:[63]
- The tumor cells are resistant to conventional therapies.
- The brain is susceptible to damage from conventional therapy.
- The brain has a limited capacity to repair itself.
- Many drugs cannot cross the blood–brain barrier to act on the tumor.
Treatment of primary brain tumors consists of palliative (symptomatic) care and therapies intended to improve survival.
Symptomatic therapy
Supportive treatment focuses on relieving symptoms and improving the patient's neurologic function. The primary supportive agents are anticonvulsants and corticosteroids.
- Historically, around 90% of patients with glioblastoma underwent anticonvulsant treatment, although only an estimated 40% of patients required this treatment. Neurosurgeons have recommended that anticonvulsants not be administered prophylactically, and should wait until a seizure occurs before prescribing this medication.[64] Those receiving phenytoin concurrent with radiation may have serious skin reactions such as erythema multiforme and Stevens–Johnson syndrome.
- Corticosteroids, usually dexamethasone, can reduce peritumoral edema (through rearrangement of the blood–brain barrier), diminishing mass effect and lowering intracranial pressure, with a decrease in headache or drowsiness.
Surgery

Surgery is the first stage of treatment of glioblastoma. An average GBM tumor contains 1011 cells, which is on average reduced to 109 cells after surgery (a reduction of 99%). Benefits of surgery include resection for a pathological diagnosis, alleviation of symptoms related to mass effect, and potentially removing disease before secondary resistance to radiotherapy and chemotherapy occurs.[65]
The greater the extent of tumor removal, the better. In retrospective analyses, removal of 98% or more of the tumor has been associated with a significantly longer healthier time than if less than 98% of the tumor is removed.[66] The chances of near-complete initial removal of the tumor may be increased if the surgery is guided by a fluorescent dye known as 5-aminolevulinic acid.[67][68] GBM cells are widely infiltrative through the brain at diagnosis, and despite a "total resection" of all obvious tumor, most people with GBM later develop recurrent tumors either near the original site or at more distant locations within the brain. Other modalities, typically radiation and chemotherapy, are used after surgery in an effort to suppress and slow recurrent disease through damaging the DNA of rapidly proliferative GBM cells.[69]
Radiotherapy

Subsequent to surgery, radiotherapy becomes the mainstay of treatment for people with glioblastoma. It is typically performed along with giving temozolomide.[10] A pivotal clinical trial carried out in the early 1970s showed that among 303 GBM patients randomized to radiation or best medical therapy, those who received radiation had a median survival more than double those who did not.[70] Subsequent clinical research has attempted to build on the backbone of surgery followed by radiation. Whole-brain radiotherapy does not improve when compared to the more precise and targeted three-dimensional conformal radiotherapy.[71] A total radiation dose of 60–65 Gy has been found to be optimal for treatment.[72]
GBM tumors are well known to contain zones of tissue exhibiting hypoxia, which are highly resistant to radiotherapy. Various approaches to chemotherapy radiosensitizers have been pursued, with limited success (As of 2016 ). (As of 2010), newer research approaches included preclinical and clinical investigations into the use of an oxygen diffusion-enhancing compound such as trans sodium crocetinate as radiosensitizers,[73] and (As of 2015) a clinical trial was underway.[74] Boron neutron capture therapy has been tested as an alternative treatment for glioblastoma, but is not in common use.
Chemotherapy
Most studies show no benefit from the addition of chemotherapy. However, a large clinical trial of 575 participants randomized to standard radiation versus radiation plus temozolomide chemotherapy showed that the group receiving temozolomide survived a median of 14.6 months as opposed to 12.1 months for the group receiving radiation alone.[10][75] This treatment regimen is now standard for most cases of glioblastoma where the person is not enrolled in a clinical trial.[76][77] Temozolomide seems to work by sensitizing the tumor cells to radiation, and appears more effective for tumors with MGMT promoter methylation.[78] High doses of temozolomide in high-grade gliomas yield low toxicity, but the results are comparable to the standard doses.[79] Antiangiogenic therapy with medications such as bevacizumab control symptoms, but do not appear to affect overall survival in those with glioblastoma.[80] The overall benefit of anti-angiogenic therapies as of 2019 is unclear.[80] In elderly people with newly diagnosed glioblastoma who are reasonably fit, concurrent and adjuvant chemoradiotherapy gives the best overall survival but is associated with a greater risk of haematological adverse events than radiotherapy alone.[81]
Immunotherapy
Phase 3 clinical trials of immunotherapy treatments for glioblastoma have largely failed.[82]
Other procedures
Alternating electric field therapy is an FDA-approved therapy for newly diagnosed[83] and recurrent glioblastoma.[84] In 2015, initial results from a phase-III randomized clinical trial of alternating electric field therapy plus temozolomide in newly diagnosed glioblastoma reported a three-month improvement in progression-free survival, and a five-month improvement in overall survival compared to temozolomide therapy alone,[85][86] representing the first large trial in a decade to show a survival improvement in this setting.[86] Despite these results, the efficacy of this approach remains controversial among medical experts.[87] However, increasing understanding of the mechanistic basis through which alternating electric field therapy exerts anti-cancer effects and results from ongoing phase-III clinical trials in extracranial cancers may help facilitate increased clinical acceptance to treat glioblastoma in the future.[88]
Prognosis
The most common length of survival following diagnosis is 10 to 13 months (although recent research points to a median survival rate of 15 months),[89][90][8] with fewer than 1–3% of people surviving longer than five years.[2][5][91] In the United States between 2012 and 2016 five-year survival was 6.8%.[5] Without treatment, survival is typically three months.[14] Complete cures are extremely rare, but have been reported.[92][93]
Increasing age (> 60 years) carries a worse prognostic risk. Death is usually due to widespread tumor infiltration with cerebral edema and increased intracranial pressure.[94]
A good initial Karnofsky performance score (KPS) and MGMT methylation are associated with longer survival.[94] A DNA test can be conducted on glioblastomas to determine whether or not the promoter of the MGMT gene is methylated. Patients with a methylated MGMT promoter have longer survival than those with an unmethylated MGMT promoter, due in part to increased sensitivity to temozolomide.[95]
Long-term benefits have also been associated with those patients who receive surgery, radiotherapy, and temozolomide chemotherapy.[94] However, much remains unknown about why some patients survive longer with glioblastoma. Age under 50 is linked to longer survival in GBM, as is 98%+ resection and use of temozolomide chemotherapy and better KPSs. A recent study confirms that younger age is associated with a much better prognosis, with a small fraction of patients under 40 years of age achieving a population-based cure. Cure is thought to occur when a person's risk of death returns to that of the normal population, and in GBM, this is thought to occur after 10 years.[93]
UCLA Neuro-oncology publishes real-time survival data for patients with this diagnosis.[96]
According to a 2003 study, GBM prognosis can be divided into three subgroups dependent on KPS, the age of the patient, and treatment.[97]
| Recursive partitioning analysis (RPA) class |
Definition | Historical Median Survival Time | Historical 1-Year Survival | Historical 3-Year Survival | Historical 5-Year Survival |
|---|---|---|---|---|---|
| III | Age < 50, KPS ≥ 90 | 17.1 months | 70% | 20% | 14% |
| IV | Age < 50, KPS < 90 | 11.2 months | 46% | 7% | 4% |
| Age ≥ 50, KPS ≥ 70, surgical removal with good neurologic function | |||||
| V + VI | Age ≥ 50, KPS ≥ 70, no surgical removal | 7.5 months | 28% | 1% | 0% |
| Age ≥ 50, KPS < 70 |
Epidemiology
About three per 100,000 people develop the disease a year,[3] although regional frequency may be much higher.[98] The frequency in England doubled between 1995 and 2015.[99]
It is the second-most common central nervous system tumor after meningioma.[15] It occurs more commonly in males than females.[2][3] Although the average age at diagnosis is 64,[2][3] in 2014, the broad category of brain cancers was second only to leukemia in people in the United States under 20 years of age.[100]
History
The term glioblastoma multiforme was introduced in 1926 by Percival Bailey and Harvey Cushing, based on the idea that the tumor originates from primitive precursors of glial cells (glioblasts), and the highly variable appearance due to the presence of necrosis, hemorrhage, and cysts (multiform).[101]
Research
Gene therapy
Gene therapy has been explored as a method to treat glioblastoma, and while animal models and early-phase clinical trials have been successful, as of 2017, all gene-therapy drugs that had been tested in phase-III clinical trials for glioblastoma had failed.[102][103][104] Scientists have developed the core–shell nanostructured LPLNP-PPT (long persistent luminescence nanoparticles. PPT refers to polyetherimide, PEG and trans-activator of transcription, and TRAIL is the human tumor necrosis factor-related apoptosis-induced ligand[105]) for effective gene delivery and tracking, with positive results. This is a TRAIL ligand that has been encoded to induce apoptosis of cancer cells, more specifically glioblastomas. Although this study was still in clinical trials in 2017, it has shown diagnostic and therapeutic functionalities, and will open great interest for clinical applications in stem-cell-based therapy.[106]
Oncolytic virotherapy
Oncolytic virotherapy is an emerging novel treatment that is under investigation both at preclinical and clinical stages. Several viruses including herpes simplex virus, adenovirus, poliovirus, and reovirus are currently being tested in phases I and II of clinical trials for glioblastoma therapy and have shown to improve overall survival.[107]
Intranasal drug delivery
Direct nose-to-brain drug delivery is being explored as a means to achieve higher, and hopefully more effective, drug concentrations in the brain.[108][109] A clinical phase-I/II study with glioblastoma patients in Brazil investigated the natural compound perillyl alcohol for intranasal delivery as an aerosol. The results were encouraging[108][110][111] and, as of 2016, a similar trial has been initiated in the United States.[112]
Cannabinoids
The efficacy of cannabinoids (cannabis derivatives) is known in oncology (through capsules of tetrahydrocannabinol (THC) or the synthetic analogue nabilone), on the one hand to combat nausea and vomiting induced by chemotherapy, on the other to stimulate appetite and lessen the sense of anguish or the actual pain.[113][114] Their ability to inhibit growth and angiogenesis in malignant gliomas in mouse models has been demonstrated.[115][116] The results of a pilot study on the use of THC in end-stage patients with recurrent glioblastoma appeared worthy of further study.[117] A potential avenue for future research rests on the discovery that cannabinoids are able to attack the neoplastic stem cells of glioblastoma in mouse models, with the result on the one hand of inducing their differentiation into more mature, possibly more "treatable" cells, and on the other hand to inhibit tumorigenesis.[118]
See also
- Glioblastoma Foundation
- List of brain tumor cases
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Current trends in the surgical management and treatment of adult glioblastoma". Annals of Translational Medicine 3 (9): 121. June 2015. doi:10.3978/j.issn.2305-5839.2015.05.10. PMID 26207249.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 "Chapter 5.16". World Cancer Report 2014. World Health Organization. 2014. ISBN 978-9283204299.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 "Nonsurgical treatment of recurrent glioblastoma". Current Oncology 22 (4): e273–e281. August 2015. doi:10.3747/co.22.2436. PMID 26300678.
- ↑ 4.0 4.1 "Temozolomide for high grade glioma". The Cochrane Database of Systematic Reviews 2013 (4): CD007415. April 2013. doi:10.1002/14651858.CD007415.pub2. PMID 23633341.
- ↑ 5.0 5.1 5.2 5.3 "CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016". Neuro-Oncology 21 (Suppl 5): v1–v100. November 2019. doi:10.1093/neuonc/noz150. PMID 31675094.
- ↑ 6.0 6.1 6.2 6.3 "Recent advances in the molecular understanding of glioblastoma". Journal of Neuro-Oncology 108 (1): 11–27. May 2012. doi:10.1007/s11060-011-0793-0. PMID 22270850.
- ↑ "Management of glioblastoma: State of the art and future directions". CA (Wiley) 70 (4): 299–312. July 2020. doi:10.3322/caac.21613. PMID 32478924.
- ↑ 8.0 8.1 "Survival comparison between glioblastoma multiforme and other incurable cancers". Journal of Clinical Neuroscience 17 (4): 417–421. April 2010. doi:10.1016/j.jocn.2009.09.004. PMID 20167494.
- ↑ "Chapter 3.8". World Cancer Report 2014. World Health Organization. 2014. ISBN 978-9283204299.
- ↑ 10.0 10.1 10.2 "Concurrent therapy to enhance radiotherapeutic outcomes in glioblastoma". Annals of Translational Medicine 4 (3): 54. February 2016. doi:10.3978/j.issn.2305-5839.2016.01.25. PMID 26904576.
- ↑ "Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma". CA 60 (3): 166–193. 2010. doi:10.3322/caac.20069. PMID 20445000.
- ↑ "Radiomic Analysis to Predict Histopathologically Confirmed Pseudoprogression in Glioblastoma Patients" (in English). Advances in Radiation Oncology 8 (1): 100916. 2022-02-06. doi:10.1016/j.adro.2022.100916. PMID 36711062.
- ↑ "Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial" (in English). The Lancet. Oncology 10 (5): 459–466. May 2009. doi:10.1016/S1470-2045(09)70025-7. PMID 19269895.
- ↑ 14.0 14.1 Neurology and clinical neuroscience. Philadelphia: Mosby Elsevier. 2007. p. 1336. ISBN 978-0323070539. https://books.google.com/books?id=EwajBQAAQBAJ&pg=PA1336.
- ↑ 15.0 15.1 "Epidemiology of Brain Tumors". Neurologic Clinics 34 (4): 981–998. November 2016. doi:10.1016/j.ncl.2016.06.014. PMID 27720005.
- ↑ Ostrom, Quinn T.; Patil, Nirav; Cioffi, Gino; Waite, Kristin; Kruchko, Carol; Barnholtz-Sloan, Jill S. (2020-10-30). "CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013-2017". Neuro-Oncology 22 (12 Suppl 2): iv1–iv96. doi:10.1093/neuonc/noaa200. ISSN 1523-5866. PMID 33123732.
- ↑ Visser, Otto; Ardanaz, Eva; Botta, Laura; Sant, Milena; Tavilla, Andrea; Minicozzi, Pamela; EUROCARE-5 Working Group (October 2015). "Survival of adults with primary malignant brain tumours in Europe; Results of the EUROCARE-5 study". European Journal of Cancer 51 (15): 2231–2241. doi:10.1016/j.ejca.2015.07.032. ISSN 1879-0852. PMID 26421825. https://pubmed.ncbi.nlm.nih.gov/26421825/.
- ↑ 18.0 18.1 18.2 18.3 18.4 "Glioblastoma multiforme: Pathogenesis and treatment". Pharmacology & Therapeutics 152: 63–82. August 2015. doi:10.1016/j.pharmthera.2015.05.005. PMID 25944528.
- ↑ "Current data and strategy in glioblastoma multiforme". Journal of Medicine and Life 2 (4): 386–393. 25 November 2009. PMID 20108752.
- ↑ Smoll NR, Brady Z, Scurrah KJ, Lee C, Berrington de González A, Mathews JD. Computed tomography scan radiation and brain cancer incidence. Neuro-Oncology. 2023 Jan 14;https://doi.org/10.1093/neuonc/noad012
- ↑ Smoll NR, Brady Z, Scurrah K, Mathews JD. Exposure to ionizing radiation and brain cancer incidence: The Life Span Study cohort. Cancer Epidemiology. 2016 Jun;42:60–5.
- ↑ "Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas". Journal of Neuropathology and Experimental Neurology 64 (6): 479–489. June 2005. doi:10.1093/jnen/64.6.479. PMID 15977639.
- ↑ "Simian virus 40 in human cancers". The American Journal of Medicine 114 (8): 675–684. June 2003. doi:10.1016/S0002-9343(03)00087-1. PMID 12798456.
- ↑ "Detection of human herpesvirus-6 variants in pediatric brain tumors: association of viral antigen in low grade gliomas". Journal of Clinical Virology 46 (1): 37–42. September 2009. doi:10.1016/j.jcv.2009.05.011. PMID 19505845.
- ↑ "Human herpesvirus 6 latent infection in patients with glioma". The Journal of Infectious Diseases 206 (9): 1394–1398. November 2012. doi:10.1093/infdis/jis513. PMID 22962688.
- ↑ "The Viral Connection to Glioblastoma". Current Infectious Disease Reports 19 (2): 5. February 2017. doi:10.1007/s11908-017-0563-z. PMID 28233187.
- ↑ "Dietary cured meat and the risk of adult glioma: a meta-analysis of nine observational studies". Journal of Environmental Pathology, Toxicology and Oncology 22 (2): 129–137. 2003. doi:10.1615/JEnvPathToxOncol.v22.i2.60. PMID 14533876.
- ↑ "Cellular phone use and brain tumor: a meta-analysis". Journal of Neuro-Oncology 86 (1): 71–78. January 2008. doi:10.1007/s11060-007-9432-1. PMID 17619826.
- ↑ "Epidemiological evidence for an association between use of wireless phones and tumor diseases". Pathophysiology 16 (2–3): 113–122. August 2009. doi:10.1016/j.pathophys.2009.01.003. PMID 19268551.
- ↑ "The cellular origin for malignant glioma and prospects for clinical advancements". Expert Review of Molecular Diagnostics 12 (4): 383–394. May 2012. doi:10.1586/erm.12.30. PMID 22616703.
- ↑ "Cell of origin for malignant gliomas and its implication in therapeutic development". Cold Spring Harbor Perspectives in Biology 7 (5): a020610. January 2015. doi:10.1101/cshperspect.a020610. PMID 25635044.
- ↑ "MRI evaluation of pathologies affecting the corpus callosum: A pictorial essay". The Indian Journal of Radiology & Imaging 23 (4): 321–332. October 2013. doi:10.4103/0971-3026.125604. PMID 24604936.
- ↑ "Histological typing of tumours of the central nervous system". Geneva: World Health Organization 21. 1979. OCLC 567810677. https://www.worldcat.org/oclc/567810677.
- ↑ "The 2007 WHO classification of tumours of the central nervous system". Acta Neuropathologica 114 (2): 97–109. August 2007. doi:10.1007/s00401-007-0243-4. PMID 17618441.
- ↑ "The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary". Acta Neuropathologica 131 (6): 803–820. June 2016. doi:10.1007/s00401-016-1545-1. PMID 27157931.
- ↑ "The 2021 WHO Classification of Tumors of the Central Nervous System: a summary". Neuro-Oncology 23 (8): 1231–1251. August 2021. doi:10.1093/neuonc/noab106. PMID 34185076.
- ↑ "WHO 2021 CNS Manual". https://academic.oup.com/neuro-oncology/article/23/8/1231/6311214.
- ↑ 38.0 38.1 "Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment". Cancer Cell 32 (1): 42–56.e6. July 2017. doi:10.1016/j.ccell.2017.06.003. PMID 28697342.
- ↑ "Genomics boosts brain-cancer work". Nature 463 (7279): 278. January 2010. doi:10.1038/463278a. PMID 20090720.
- ↑ "Biomarkers and therapeutic advances in glioblastoma multiforme". Asia-Pacific Journal of Clinical Oncology 14 (1): 40–51. February 2018. doi:10.1111/ajco.12756. PMID 28840962.

- ↑ 41.0 41.1 "Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1". Cancer Cell 17 (1): 98–110. January 2010. doi:10.1016/j.ccr.2009.12.020. PMID 20129251.
- ↑ "Genomics illuminates a deadly brain cancer". JAMA 303 (10): 925–927. March 2010. doi:10.1001/jama.2010.236. PMID 20215599.
- ↑ "Mutational profiling of kinases in glioblastoma". BMC Cancer 14 (1): 718. September 2014. doi:10.1186/1471-2407-14-718. PMID 25256166.
- ↑ 44.0 44.1 "The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone". Neuro-Oncology 16 (9): 1263–1273. September 2014. doi:10.1093/neuonc/nou005. PMID 24510240.
- ↑ 45.0 45.1 45.2 "The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation". Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1846 (2): 326–341. December 2014. doi:10.1016/j.bbcan.2014.05.004. PMID 24880135.
- ↑ "MGMT gene silencing and benefit from temozolomide in glioblastoma". The New England Journal of Medicine 352 (10): 997–1003. March 2005. doi:10.1056/NEJMoa043331. PMID 15758010.
- ↑ Furnari, Frank B.; Fenton, Tim; Bachoo, Robert M.; Mukasa, Akitake; Stommel, Jayne M.; Stegh, Alexander; Hahn, William C.; Ligon, Keith L. et al. (2007-11-01). "Malignant astrocytic glioma: genetics, biology, and paths to treatment". Genes & Development 21 (21): 2683–2710. doi:10.1101/gad.1596707. ISSN 0890-9369. PMID 17974913. http://dx.doi.org/10.1101/gad.1596707.
- ↑ Greenberg, Mark S. (2016). Handbook of Neurosurgery. doi:10.1055/b-006-149702. ISBN 978-1-62623-242-6. http://dx.doi.org/10.1055/b-006-149702.
- ↑ "Stem cell-related "self-renewal" signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma". Journal of Clinical Oncology 26 (18): 3015–3024. June 2008. doi:10.1200/JCO.2007.15.7164. PMID 18565887.
- ↑ "Making a tumour's bed: glioblastoma stem cells and the vascular niche". Nature Reviews. Cancer 7 (10): 733–736. October 2007. doi:10.1038/nrc2246. PMID 17882276.
- ↑ "Multilayered Heterogeneity of Glioblastoma Stem Cells: Biological and Clinical Significance". Stem Cells Heterogeneity in Cancer. Advances in Experimental Medicine and Biology. 1139. 2019. pp. 1–21. doi:10.1007/978-3-030-14366-4_1. ISBN 978-3-030-14365-7.
- ↑ "Chemotherapeutic Drugs: DNA Damage and Repair in Glioblastoma". Cancers 9 (6): 57. May 2017. doi:10.3390/cancers9060057. PMID 28587121.
- ↑ "Ion channels in glioblastoma". ISRN Neurology 2011: 590249. 2011. doi:10.5402/2011/590249. PMID 22389824.
- ↑ "A systematic review of microRNA in glioblastoma multiforme: micro-modulators in the mesenchymal mode of migration and invasion". Molecular Neurobiology 47 (1): 131–144. February 2013. doi:10.1007/s12035-012-8349-7. PMID 23054677.
- ↑ "MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells". Molecular Cell 37 (5): 620–632. March 2010. doi:10.1016/j.molcel.2010.02.018. PMID 20227367.
- ↑ "The functional synergism of microRNA clustering provides therapeutically relevant epigenetic interference in glioblastoma". Nature Communications 10 (1): 442. January 2019. doi:10.1038/s41467-019-08390-z. PMID 30683859. Bibcode: 2019NatCo..10..442B.
- ↑ 57.0 57.1 "The glioblastoma vasculature as a target for cancer therapy". Biochemical Society Transactions 42 (6): 1647–1652. December 2014. doi:10.1042/BST20140278. PMID 25399584.
- ↑ "Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers". Journal of Clinical Oncology 31 (17): 2205–2218. June 2013. doi:10.1200/JCO.2012.46.3653. PMID 23669226.
- ↑ "Patterns of contrast enhancement in the brain and meninges". Radiographics 27 (2): 525–551. 2007. doi:10.1148/rg.272065155. PMID 17374867.
- ↑ "Liquid biopsy and glioblastoma". Exploration of Targeted Anti-Tumor Therapy 4 (1): 28–41. February 2023. doi:10.37349/etat.2023.00121. PMID 36937320.
- ↑ "Isocitrate Dehydrogenase Mutations Are Associated with Different Expression and DNA Methylation Patterns of OLIG2 in Adult Gliomas". Journal of Neuropathology and Experimental Neurology 81 (9): 707–716. August 2022. doi:10.1093/jnen/nlac059. PMID 35856894.
- ↑ "Gliomas Prevention". Ohio State University. https://cancer.osu.edu/for-patients-and-caregivers/learn-about-cancers-and-treatments/cancers-conditions-and-treatment/cancer-types/gliomas/prevention.
- ↑ "Interstitial chemotherapy for malignant gliomas: the Johns Hopkins experience". Journal of Neuro-Oncology 83 (1): 61–70. May 2007. doi:10.1007/s11060-006-9303-1. PMID 17171441.
- ↑ "Antiepileptic therapy in patients with central nervous system malignancies". Current Neurology and Neuroscience Reports 6 (4): 311–318. July 2006. doi:10.1007/s11910-006-0024-9. PMID 16822352.
- ↑ "Angiogenesis in glioblastoma". The New England Journal of Medicine 369 (16): 1561–1563. October 2013. doi:10.1056/NEJMcibr1309402. PMID 24131182.
- ↑ "A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival". Journal of Neurosurgery 95 (2): 190–198. August 2001. doi:10.3171/jns.2001.95.2.0190. PMID 11780887.
- ↑ "Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial". The Lancet. Oncology 7 (5): 392–401. May 2006. doi:10.1016/S1470-2045(06)70665-9. PMID 16648043.
- ↑ "Outcomes after combined use of intraoperative MRI and 5-aminolevulinic acid in high-grade glioma surgery". Neuro-Oncology 17 (12): 1560–1567. December 2015. doi:10.1093/neuonc/nov049. PMID 25858636.
- ↑ "DDRugging glioblastoma: understanding and targeting the DNA damage response to improve future therapies". Molecular Oncology 16 (1): 11–41. January 2022. doi:10.1002/1878-0261.13020. PMID 34036721.
- ↑ "Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial". Journal of Neurosurgery 49 (3): 333–343. September 1978. doi:10.3171/jns.1978.49.3.0333. PMID 355604.
- ↑ "Multifocal glioblastoma multiforme: prognostic factors and patterns of progression". International Journal of Radiation Oncology, Biology, Physics 69 (3): 820–824. November 2007. doi:10.1016/j.ijrobp.2007.03.045. PMID 17499453.
- ↑ "Increasing radiation dose intensity using hyperfractionation in patients with malignant glioma. Final report of a prospective phase I-II dose response study". Journal of Neuro-Oncology 14 (1): 63–72. September 1992. doi:10.1007/BF00170946. PMID 1335044.
- ↑ "Improving the radiosensitivity of radioresistant and hypoxic glioblastoma". Future Oncology 6 (10): 1591–1601. October 2010. doi:10.2217/fon.10.123. PMID 21062158.
- ↑ Clinical trial number NCT01465347 for "Safety and Efficacy Study of Trans Sodium Crocetinate (TSC) With Concomitant Radiation Therapy and Temozolomide in Newly Diagnosed Glioblastoma (GBM)" at ClinicalTrials.gov, accessed 2016-02-01
- ↑ "Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma". The New England Journal of Medicine 352 (10): 987–996. March 2005. doi:10.1056/NEJMoa043330. PMID 15758009.
- ↑ "Radiotherapy with Concurrent and Adjuvant Temozolomide: A New Standard of Care for Glioblastoma Multiforme". Progress in Neurotherapeutics and Neuropsychopharmacology 1 (1): 37–52. 2006. doi:10.1017/S1748232105000054. ISBN 978-0-521-86253-0. https://books.google.com/books?id=mDhxMg5UXvIC&pg=PA37.
- ↑ "Temozolomide Plus Radiation Helps Brain Cancer – National Cancer Institute". http://www.cancer.gov/clinicaltrials/results/glioblastoma0604.
- ↑ "Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma". Journal of Neuro-Oncology 82 (1): 81–83. March 2007. doi:10.1007/s11060-006-9241-y. PMID 16944309.
- ↑ "Dose-intensity temozolomide after concurrent chemoradiotherapy in operated high-grade gliomas". Journal of Neuro-Oncology 90 (3): 315–319. December 2008. doi:10.1007/s11060-008-9663-9. PMID 18688571.
- ↑ 80.0 80.1 "Anti-angiogenic therapy for high-grade glioma". The Cochrane Database of Systematic Reviews 2018 (11): CD008218. November 2018. doi:10.1002/14651858.CD008218.pub4. PMID 30480778. "The use of anti-angiogenic therapy does not significantly improve overall survival in newly diagnosed people with glioblastoma. Thus, there is insufficient evidence to support the use of anti-angiogenic therapy for people with newly diagnosed glioblastoma at this time.".
- ↑ "Treatment of newly diagnosed glioblastoma in the elderly: a network meta-analysis". The Cochrane Database of Systematic Reviews 2020 (3): CD013261. March 2020. doi:10.1002/14651858.cd013261.pub2. PMID 32202316.
- ↑ "Challenges in glioblastoma immunotherapy: mechanisms of resistance and therapeutic approaches to overcome them". British Journal of Cancer 127 (6): 976–987. October 2022. doi:10.1038/s41416-022-01864-w. PMID 35662275.
- ↑ "FDA approves expanded indication for medical device to treat a form of brain cancer". https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm465744.htm.
- ↑ "FDA approval letter – NovoTTF-100A System". http://www.accessdata.fda.gov/cdrh_docs/pdf10/p100034a.pdf.
- ↑ "Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial". JAMA 314 (23): 2535–2543. December 2015. doi:10.1001/jama.2015.16669. PMID 26670971.
- ↑ 86.0 86.1 "Alternating Electric Fields for the Treatment of Glioblastoma". JAMA 314 (23): 2511–2513. December 2015. doi:10.1001/jama.2015.16701. PMID 26670969.
- ↑ "TTFields: where does all the skepticism come from?". Neuro-Oncology 18 (3): 303–305. March 2016. doi:10.1093/neuonc/now012. PMID 26917587.
- ↑ "Tumour treating fields therapy for glioblastoma: current advances and future directions". British Journal of Cancer 124 (4): 697–709. February 2021. doi:10.1038/s41416-020-01136-5. PMID 33144698.
- ↑ "Epidemiology of Glioblastoma Multiforme-Literature Review". Cancers 14 (10): 2412. May 2022. doi:10.3390/cancers14102412. PMID 35626018.
- ↑ "Improved survival time trends for glioblastoma using the SEER 17 population-based registries". Journal of Neuro-Oncology 107 (1): 207–212. March 2012. doi:10.1007/s11060-011-0738-7. PMID 21984115.
- ↑ "Long-term survival of patients with glioblastoma multiforme (GBM)". Journal of Clinical Neuroscience 20 (5): 670–675. May 2013. doi:10.1016/j.jocn.2012.05.040. PMID 23352352.
- ↑ "A very rare case report of long-term survival: A patient operated on in 1994 of glioblastoma multiforme and currently in perfect health". International Journal of Surgery Case Reports 33: 41–43. 2017-02-20. doi:10.1016/j.ijscr.2017.02.025. PMID 28273605.
- ↑ 93.0 93.1 "The cure fraction of glioblastoma multiforme". Neuroepidemiology 39 (1): 63–69. 2012. doi:10.1159/000339319. PMID 22776797.
- ↑ 94.0 94.1 94.2 "Long-term survival with glioblastoma multiforme". Brain 130 (Pt 10): 2596–2606. October 2007. doi:10.1093/brain/awm204. PMID 17785346.
- ↑ "Frequent hypermethylation of the DNA repair gene MGMT in long-term survivors of glioblastoma multiforme". Journal of Neuro-Oncology 83 (1): 91–93. May 2007. doi:10.1007/s11060-006-9292-0. PMID 17164975.
- ↑ "University of California, Los Angeles Neuro-Oncology : How Our Patients Perform : Glioblastoma Multiforme [GBM"]. http://www.neurooncology.ucla.edu/Performance/GlioblastomaMultiforme.aspx.. Neurooncology.ucla.edu. Retrieved on 2010-10-19.
- ↑ "Reexamining the radiation therapy oncology group (RTOG) recursive partitioning analysis (RPA) for glioblastoma multiforme (GBM) patients". International Journal of Radiation Oncology, Biology, Physics 57 (2): S135–36. 2003. doi:10.1016/S0360-3016(03)00843-5.
- ↑ "Geographic Variations in the Incidence of Glioblastoma and Prognostic Factors Predictive of Overall Survival in US Adults from 2004-2013". Frontiers in Aging Neuroscience 9: 352. 2017. doi:10.3389/fnagi.2017.00352. PMID 29163134.
- ↑ "Brain Tumours: Rise in Glioblastoma Multiforme Incidence in England 1995-2015 Suggests an Adverse Environmental or Lifestyle Factor". Journal of Environmental and Public Health 2018: 7910754. 2018. doi:10.1155/2018/7910754. PMID 30034480.
- ↑ "Geographic Variation in Pediatric Cancer Incidence - United States, 2003-2014" (in en-us). MMWR. Morbidity and Mortality Weekly Report 67 (25): 707–713. June 2018. doi:10.15585/mmwr.mm6725a2. PMID 29953430.
- ↑ Bailey & Cushing: Tumors of the Glioma Group. JB Lippincott, Philadelphia, 1926.[page needed]
- ↑ "Insights into molecular therapy of glioma: current challenges and next generation blueprint". Acta Pharmacologica Sinica 38 (5): 591–613. May 2017. doi:10.1038/aps.2016.167. PMID 28317871.
- ↑ "The art of gene therapy for glioma: a review of the challenging road to the bedside". Journal of Neurology, Neurosurgery, and Psychiatry 84 (2): 213–222. February 2013. doi:10.1136/jnnp-2012-302946. PMID 22993449.
- ↑ "The status of gene therapy for brain tumors". Expert Opinion on Biological Therapy 7 (2): 197–208. February 2007. doi:10.1517/14712598.7.2.197. PMID 17250458.
- ↑ "Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs". Genes (MDPI AG) 1 (3): 413–426. November 2010. doi:10.3390/chemosensors8040117. PMID 24710095.
- ↑ "A Dual-Functional Persistently Luminescent Nanocomposite Enables Engineering of Mesenchymal Stem Cells for Homing and Gene Therapy of Glioblastoma". Advanced Functional Materials 27 (11): 1604992. March 2017. doi:10.1002/adfm.201604992.
- ↑ "Oncolytic Viruses for Malignant Glioma: On the Verge of Success?". Viruses 13 (7): 1294. July 2021. doi:10.3390/v13071294. PMID 34372501.
- ↑ 108.0 108.1 "Formulations for Intranasal Delivery of Pharmacological Agents to Combat Brain Disease: A New Opportunity to Tackle GBM?". Cancers 5 (3): 1020–1048. August 2013. doi:10.3390/cancers5031020. PMID 24202332.
- ↑ "Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: an excellent platform for brain targeting". Expert Opinion on Drug Delivery 10 (7): 957–972. July 2013. doi:10.1517/17425247.2013.790887. PMID 23586809.
- ↑ "A systematic review of inhaled intranasal therapy for central nervous system neoplasms: an emerging therapeutic option". Journal of Neuro-Oncology 116 (3): 437–446. February 2014. doi:10.1007/s11060-013-1346-5. PMID 24398618.
- ↑ "Preclinical development and clinical use of perillyl alcohol for chemoprevention and cancer therapy". American Journal of Cancer Research 5 (5): 1580–1593. 2015. PMID 26175929.
- ↑ Clinical trial number NCT02704858 for "Safety and Efficacy Study in Recurrent Grade IV Glioma" at ClinicalTrials.gov
- ↑ "Cannabinoids and cancer: causation, remediation, and palliation". The Lancet. Oncology 6 (1): 35–42. January 2005. doi:10.1016/S1470-2045(04)01711-5. PMID 15629274.
- ↑ "Cannabinoids: potential anticancer agents". Nature Reviews. Cancer 3 (10): 745–755. October 2003. doi:10.1038/nrc1188. PMID 14570037.
- ↑ "Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines". The Journal of Pharmacology and Experimental Therapeutics 308 (3): 838–845. March 2004. doi:10.1124/jpet.103.061002. PMID 14617682.
- ↑ "Inhibition of tumor angiogenesis by cannabinoids". FASEB Journal 17 (3): 529–531. March 2003. doi:10.1096/fj.02-0795fje. PMID 12514108.
- ↑ "A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme". British Journal of Cancer 95 (2): 197–203. July 2006. doi:10.1038/sj.bjc.6603236. PMID 16804518.
- ↑ "Cannabinoids induce glioma stem-like cell differentiation and inhibit gliomagenesis". The Journal of Biological Chemistry 282 (9): 6854–6862. March 2007. doi:10.1074/jbc.M608900200. PMID 17202146.
External links
- Information about glioblastoma from the American Brain Tumor Association
| Classification | |
|---|---|
| External resources |
 |


