Biology:Metabolic imprinting
Metabolic imprinting refers to the long-term physiological and metabolic effects that an offspring's prenatal and postnatal environments have on them.[1] Perinatal nutrition has been identified as a significant factor in determining an offspring's likelihood of it being predisposed to developing cardiovascular disease, obesity, and type 2 diabetes amongst other conditions.[2]
During pregnancy, maternal glucose can cross the blood-placental barrier[3] meaning maternal hyperglycaemia is associated with foetal hyperglycaemia.[4] Despite maternal glucose being able to cross the blood-placental barrier, maternal insulin is not able and the foetus has to make its own.[5][6] As a result, if a mother is hyperglycaemic the foetus is likely to be hyperinsulinaemic which leads to it having increased levels of growth and adiposity.[4]
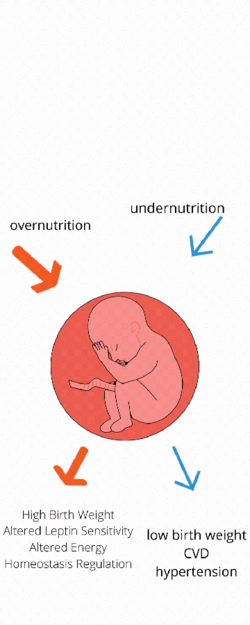
Maternal undernutrition
Maternal undernutrition has been linked with low birth weight and also a number of diseases, including Cardiovascular disease, stroke, hypertension and diabetes.[7] When a foetus is in the womb and is not receiving sufficient nutrition, it can adapt to prioritize organ growth and increased metabolic efficiency to prepare itself for life in an energy deficient environment. Postnatally, when given the correct nutrition, babies exhibit ‘catch up growth’, potentially leading to obesity and other related complications. Studies based around restricting animals food intake throughout gestation have discovered that a reduction of just 30% of normal intake can cause low birth weight and increase sensitivity to high-fat-diet induced obesity.[8]
In animal models, intrauterine undernutrition has been shown to be associated with hypertension later in life. This is because the formation of the kidneys is inhibited, which decreases filtration and flow rate through the nephrons, leading to increased blood pressure.[9]
More extreme prenatal conditions such as famine have been shown to have effects on the neurodevelopment of a foetus.[7] After the Dutch Famine of the winter of 1944–1945, it was found that the risk of schizophrenia was significantly higher in those conceived at the height of the famine, as was the prevalence of schizoid personality.[10]
Maternal over-nutrition
Maternal overnutrition can have detrimental effects on the health of the offspring later in life. This area is less well studied and understood but some progress has been made in identifying specific genes that are affected.[7] Studies have investigated hypermethylation of DNA and found it to be higher in obese mothers to those of a healthy BMI.[11] More specific studies have investigated Leptin (LEP) as a possible gene which is altered via metabolic imprinting in response to overnutrition in utero, and found hypermethylation of LEP in the placenta of those born to overly nourished mothers.[12] This hypermethylation has been found to cause changes in the levels of circulating Leptin, as well as to leptin sensitivity and the development of neural circuits involved in the control of homeostasis which causes the higher risk of metabolic disease.
Upon investigation it was found that a mother who was obese before conception was likely to have a higher level of placental LEP than the placenta of a mother of a healthy weight.[12] One strategy for overcoming obesity is the use of gastric bypass and other such surgeries, while this does not entirely alleviate the risk of altered metabolic imprinting it has been found that siblings born post maternal surgery are less likely to have as high body fat percentages than over nutrition as siblings born before the surgery.[13]
Paternal overnutrition can also have a detrimental effect and new-borns have shown changes in methylation of DNA generally, with substantial hypomethylation at the gene Insulin-like Growth factor 2 (IGF2). However, this topic is much less studied than maternal nutrition.[13]
Maternal/gestational diabetes
An increase in certain hormones such as oestrogen, progesterone, human placental lactogen, human placental growth hormone and cortisol during the second and third trimester of pregnancy cause an increase in insulin resistance. This increase in insulin resistance and following increase in insulin secretion ensures that the foetus develops a normal glucose tolerance.[14] Gestational Diabetes Mellitus (GDM) arises when beta cells do not secrete enough insulin to adopt to the insulin resistance triggered by pregnancy, which leads to mild hyperglycaemia.[15]
Although the mechanisms are still largely unknown, foetus exposure to GDM and maternal diabetes has been shown to lead to lifelong metabolic complications because of metabolic imprinting. The risk of Type II diabetes developing in offspring is significantly higher in offspring where the mother was diagnosed with Type II diabetes before pregnancy rather than after. In addition, the age at which offspring are diagnosed with Type 2 diabetes is significantly younger in offspring exposed to maternal diabetes/GDM than those who are not. It is suggested that this is a result of DNA methylation during foetal development.[14]
References
- ↑ "Metabolic imprinting in obesity". Forum of Nutrition 63: 186–194. 2010. doi:10.1159/000264406. ISBN 978-3-8055-9300-7. PMID 19955786.
- ↑ "Embryonic and fetal programming of physiological disorders in adulthood". Birth Defects Research Part C: Embryo Today: Reviews 72 (4): 300–12. December 2004. doi:10.1002/bdrc.20029. PMID 15662709.
- ↑ "Functions of the placenta.". Anaesthesia & Intensive Care Medicine 15 (3): 136–9. March 2014. doi:10.1016/j.mpaic.2014.01.004.
- ↑ 4.0 4.1 "The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study". International Journal of Gynaecology and Obstetrics 78 (1): 69–77. July 2002. doi:10.1016/s0020-7292(02)00092-9. PMID 12113977.
- ↑ "Fetal origins of obesity". Obesity Research 11 (4): 496–506. April 2003. doi:10.1038/oby.2003.69. PMID 12690076.
- ↑ "Banting Lecture 1980. Of pregnancy and progeny". Diabetes 29 (12): 1023–35. December 1980. doi:10.2337/diab.29.12.1023. PMID 7002669.
- ↑ 7.0 7.1 7.2 "Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 361 (1471): 1107–21. July 2006. doi:10.1098/rstb.2006.1851. PMID 16815795.
- ↑ "The obesity epidemic: metabolic imprinting on genetically susceptible neural circuits". Obesity Research 8 (4): 342–7. July 2000. doi:10.1038/oby.2000.41. PMID 10933311.
- ↑ "Metabolic imprinting, programming and epigenetics - a review of present priorities and future opportunities". The British Journal of Nutrition 104 (S1): S1-25. July 2010. doi:10.1017/s0007114510003338. PMID 20929595.
- ↑ "Neurodevelopmental disorders after prenatal famine: The story of the Dutch Famine Study". American Journal of Epidemiology 147 (3): 213–6. February 1998. doi:10.1093/oxfordjournals.aje.a009439. PMID 9482494.; "Erratum: Neurodevelopmental disorders after prenatal famine: The story of the Dutch Famine Study". American Journal of Epidemiology 148 (11): 1135. doi:10.1093/oxfordjournals.aje.a009597.
- ↑ "Effects of maternal nutrition on the expression of genomic imprinted genes in ovine fetuses". Epigenetics 13 (8): 793–807. 2018-08-03. doi:10.1080/15592294.2018.1503489. PMID 30051747.
- ↑ 12.0 12.1 "Impact of Early Nutrition, Physical Activity and Sleep on the Fetal Programming of Disease in the Pregnancy: A Narrative Review". Nutrients 12 (12): 3900. December 2020. doi:10.3390/nu12123900. PMID 33419354.
- ↑ 13.0 13.1 "Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: A systematic review". Diabetes & Metabolic Syndrome. SI: Online Supplement - 2 11 (Suppl 2): S655–S662. December 2017. doi:10.1016/j.dsx.2017.04.021. PMID 28533070.
- ↑ 14.0 14.1 "Short- and long-term consequences for offspring exposed to maternal diabetes: a review". The Journal of Maternal-Fetal & Neonatal Medicine 32 (4): 687–694. February 2019. doi:10.1080/14767058.2017.1387893. PMID 28969466.
- ↑ "Gestational diabetes mellitus". Nature Reviews. Disease Primers 5 (1): 47. July 2019. doi:10.1038/s41572-019-0098-8. PMID 31296866.
 |