Biology:Miravirsen
 | |
| Clinical data | |
|---|---|
| Other names | SPC3649 |
| Routes of administration | Intravenous or subcutaneous injection |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C151H185N49O83P14S14 |
| Molar mass | 4896.87 g·mol−1 |
| |
Miravirsen (INN; codenamed SPC3649) is an experimental drug for the treatment of hepatitis C, being developed by Santaris Pharma. As of 2017 it was in Phase II clinical trials.[1]
Miravirsen had been given by subcutaneous injection in early clinical trials as of 2017.[1] It is antisense to a human microRNA called miR-122. miR-122 ferries an argonaute protein to 5'-UTR region of viral RNA, where it binds, protecting the RNA from being destroyed by normally present nucleases; by binding to miR-122, miravirsen removes that protection and the virus RNA can be destroyed.[1] There is some evidence that the 5'-UTR region mutates under repeated exposure to miravirsen.[1]
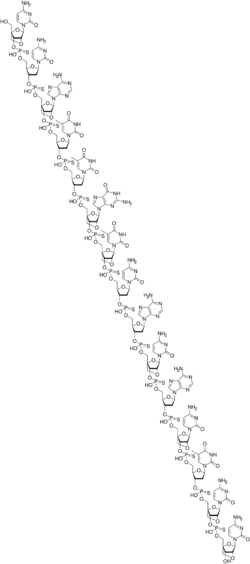
Miravirsen is a modified oligonucleotide consisting of a chain of 15 nucleotides, the base sequence of which is designed to selectively bind to miR-122.[1][2] Seven of the 15 sugar units are deoxyriboses, and the other eight are riboses with an additional bridge between the 2' oxygen and the 4' carbon atoms; this makes the molecule a locked nucleic acid. Furthermore, the phosphate units have been replaced by thiophosphates.[2]
The complete base sequence is
mC*-dC-A*-dT-dT-G*-mU*-dC-dA-mC*-dA-mC*-dT-mC*-mC*[d= 2'-deoxy,m= 5-methyl,*= 2'-O,4'-C-methylene, i.e. bridged or "locked" sugar]
with 3'→5' thiophosphate linkages.[2]
Chemical structure of a single nucleoside (sugar plus base) of a locked nucleic acid
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "The Race of 10 Synthetic RNAi-Based Drugs to the Pharmaceutical Market". Pharmaceutical Research 34 (7): 1339–1363. July 2017. doi:10.1007/s11095-017-2134-2. PMID 28389707.
- ↑ 2.0 2.1 2.2 "Miravirsen". PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/56843415.
 |


