Biology:Monoallelic gene expression
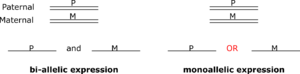
Monoallelic gene expression (MAE) is the phenomenon of the gene expression, when only one of the two gene copies (alleles) is actively expressed (transcribed), while the other is silent.[1][2][3] Diploid organisms bear two homologous copies of each chromosome (one from each parent), a gene can be expressed from both chromosomes (biallelic expression) or from only one (monoallelic expression).[4] MAE can be Random monoallelic expression (RME) or Constitutive monoallelic expression (constitutive). Constitutive monoallelic expression occurs from the same specific allele throughout the whole organism or tissue, as a result of genomic imprinting.[5] RME is a broader class of monoallelic expression, which is defined by random allelic choice in somatic cells, so that different cells of the multi-cellular organism express different alleles.
Constitutive monoallelic gene expression
Random monoallelic gene expression (RME)
X-chromosome inactivation (XCI), is the most striking and well-studied example of RME. XCI leads to the transcriptional silencing of one of the X chromosomes in female cells, which results in expression of the genes that located on the other, remaining active X chromosome. XCI is critical for balanced gene expression in female mammals. The allelic choice of XCI by individual cells takes place randomly in epiblasts of the preimplantation embryo,[6] which leads to mosaic gene expression of the paternal and maternal X chromosome in female tissues.[7][8] XCI is a chromosome-wide monoallelic expression, that includes expression of all genes that are located on X chromosome, in contrast to autosomal RME (aRME) that relates to single genes that are interspersed over the genome. aRME's can be fixed[9] or dynamic, depending whether or not the allele-specific expression is conserved in daughter cells after mitotic cell division.
Types of aRME
Fixed aRME are established either by silencing of one allele that previously has been biallelically expressed, or by activation of a single allele from previously silent gene. Expression activation of the silent allele is coupled with a feedback mechanism that prevents expression of the second allele. Another scenario is also possible due to limited time-window of low-probability initiation, that could lead to high frequencies of cells with single-allele expression. It is estimated that 2[10][11]-10[12]% of all genes are fixed aRME. Studies of fixed aRME require either expansion of monoclonal cultures or lineage-traced in vivo or in vitro cells that are mitotically.
Dynamic aRME occurs as a consequence of stochastic allelic expression. Transcription happens in bursts, which results in RNA molecules being synthesized from each allele separately. So over time, both alleles have a probability to initiate transcription. Transcriptional bursts are allelically stochastic, and lead to either maternal or paternal allele being accumulated in the cell. The gene transcription burst frequency and intensity combined with RNA-degradation rate form the shape of RNA distribution at the moment of observation and thus whether the gene is bi- or monoallelic. Studies that distinguish fixed and dynamic aRME require single-cell analyses of clonally related cells.[13]
Mechanisms of aRME
Allelic exclusion is a process of gene expression when one allele is expressed and the other one kept silent. Two most studied cases of allelic exclusion are monoallelic expression of immunoglobulins in B and T cells[14][15][16] and olfactory receptors in sensory neurons.[17] Allelic exclusion is cell-type specific (as opposed to organism-wide XCI), which increases intercellular diversity, thus specificity towards certain antigens or odors.
Allele-biased expression is skewed expression level of one allele over the other, but both alleles are still expressed (in contrast to allelic exclusion). This phenomenon is often observed in cells of immune function[18][19]
Methods of detection
Methods of MAE detection are based on the difference between alleles, which can be distinguished either by the sequence of expressed mRNA or protein structure. Methods of MAE detection can be divided into single gene or whole genome MAE analysis. Whole genome MAE analysis cannot be performed based on protein structure yet, so these are completely NGS based techniques.
Single-gene analysis
| Methods of detection | Synopsis |
|---|---|
| RT-qPCR | can be used to detect RME by using allele specific primers, SNP-sensitive hybridization probes or allele-specific restriction sites. Can be used for single cells or clonal cell population. |
| Nascent RNA FISH | visualizes nascent(which is currently being synthesized) RNA in situ . Read-out is one, two or zero fluorescent dots, which indicates mono-,di-allelic or no expression respectfully at single cell resolution. |
| Cell sorting | if the gene is a surface protein, and there is the allele-specific antibody, this technique can be used to detect presence or absence of fixed or dynamic RME by running the same cell over the time. Single cell resolution. |
| Live cell imaging | results in expression dynamics over time. Requires the insertion of allele-specific fluorescent protein tag (for example GFP), in order to detect signal. |
Genome-wide analysis
| Methods of detection | Synopsis |
|---|---|
| SNP-sensitive microarrays | can be used to give an estimate fixed RME of predefined set of transcripts for clonally expanded cell populations |
| RNA-seq | similarly to the previous method gives and estimate of fixed RME for clonally expanded cell populations, but for all transcripts. |
| Single-cell RNA sequencing | similar to the previous methods, but superior. Since, gives an opportunity for single-cell analysis. If multiple clonally related cells are analysed, can distinguish between fixed and dynamic RME's.[20] |
References
- ↑ "Monoallelic Gene Expression in Mammals". Annual Review of Genetics 50 (1): 317–327. November 2016. doi:10.1146/annurev-genet-120215-035120. PMID 27893959.
- ↑ "Parallels between Mammalian Mechanisms of Monoallelic Gene Expression". Trends in Genetics 34 (12): 954–971. December 2018. doi:10.1016/j.tig.2018.08.005. PMID 30217559.
- ↑ "Monoallelic gene expression and its mechanisms". Current Opinion in Plant Biology 14 (5): 608–13. October 2011. doi:10.1016/j.pbi.2011.07.001. PMID 21807553.
- ↑ "ASPsiRNA: A Resource of ASP-siRNAs Having Therapeutic Potential for Human Genetic Disorders and Algorithm for Prediction of Their Inhibitory Efficacy". G3 7 (9): 2931–2943. September 2017. doi:10.1534/g3.117.044024. PMID 28696921.
- ↑ "Distinct physiological and behavioural functions for parental alleles of imprinted Grb10". Nature 469 (7331): 534–8. January 2011. doi:10.1038/nature09651. PMID 21270893. Bibcode: 2011Natur.469..534G.
- ↑ "Sequential X chromosome inactivation coupled with cellular differentiation in early mouse embryos". Nature 281 (5729): 311–3. September 1979. doi:10.1038/281311a0. PMID 551278. Bibcode: 1979Natur.281..311M.
- ↑ "An X-linked GFP transgene reveals unexpected paternal X-chromosome activity in trophoblastic giant cells of the mouse placenta". Genesis 29 (3): 133–40. March 2001. doi:10.1002/gene.1016. PMID 11252054.
- ↑ "Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease". Neuron 81 (1): 103–19. January 2014. doi:10.1016/j.neuron.2013.10.051. PMID 24411735.
- ↑ "Widespread monoallelic expression on human autosomes". Science 318 (5853): 1136–40. November 2007. doi:10.1126/science.1148910. PMID 18006746. Bibcode: 2007Sci...318.1136G.
- ↑ "Stochastic choice of allelic expression in human neural stem cells". Stem Cells 30 (9): 1938–47. September 2012. doi:10.1002/stem.1155. PMID 22714879. http://repository.essex.ac.uk/11040/1/1155_ftp.pdf.
- ↑ "Transcriptome-wide survey of mouse CNS-derived cells reveals monoallelic expression within novel gene families". PLOS ONE 7 (2): e31751. 2012. doi:10.1371/journal.pone.0031751. PMID 22384067. Bibcode: 2012PLoSO...731751L.
- ↑ "Autosomal monoallelic expression in the mouse". Genome Biology 13 (2): R10. February 2012. doi:10.1186/gb-2012-13-2-r10. PMID 22348269.
- ↑ "Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells". Science 343 (6167): 193–6. January 2014. doi:10.1126/science.1245316. PMID 24408435. Bibcode: 2014Sci...343..193D.
- ↑ "Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues". The Journal of Experimental Medicine 122 (5): 853–76. November 1965. doi:10.1084/jem.122.5.853. PMID 4159057.
- ↑ "Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions". Proceedings of the National Academy of Sciences of the United States of America 73 (10): 3628–32. October 1976. doi:10.1073/pnas.73.10.3628. PMID 824647. Bibcode: 1976PNAS...73.3628H.
- ↑ "Antigen receptor allelic exclusion: an update and reappraisal". Journal of Immunology 185 (7): 3801–8. October 2010. doi:10.4049/jimmunol.1001158. PMID 20858891.
- ↑ "Allelic inactivation regulates olfactory receptor gene expression". Cell 78 (5): 823–34. September 1994. doi:10.1016/S0092-8674(94)90562-2. PMID 8087849.
- ↑ "Expression of natural killer receptor alleles at different Ly49 loci occurs independently and is regulated by major histocompatibility complex class I molecules". The Journal of Experimental Medicine 193 (3): 307–15. February 2001. doi:10.1084/jem.193.3.307. PMID 11157051.
- ↑ "Probabilistic regulation in TH2 cells accounts for monoallelic expression of IL-4 and IL-13". Immunity 23 (1): 89–99. July 2005. doi:10.1016/j.immuni.2005.05.008. PMID 16039582.
- ↑ "Random monoallelic expression of autosomal genes: stochastic transcription and allele-level regulation". Nature Reviews. Genetics 16 (11): 653–64. November 2015. doi:10.1038/nrg3888. PMID 26442639.
External links
- Singer-Sam, Judith (2010). "Monoallelic Expression". Nature Education 3 (3): 1. https://www.nature.com/scitable/topicpage/monoallelic-expression-8813275.
 |