Biology:NCAPH
 Generic protein structure example |
Condensin complex subunit 2 also known as chromosome-associated protein H (CAP-H) or non-SMC condensin I complex subunit H (NCAPH) is a protein that in humans is encoded by the NCAPH gene.[1][2] CAP-H is a subunit of condensin I, a large protein complex involved in chromosome condensation. Abnormal expression of NCAPH may be linked to various types of carcinogenesis as a prognostic indicator.[3]
Function
CAP-H is a member of the barr protein family and a regulatory subunit of the condensin complex. This complex is required for the conversion of interphase chromatin into condensed chromosomes.[3] CAP-H is associated with mitotic chromosomes, except during the early phase of chromosome condensation. During interphase, the protein has a distinct punctate nucleolar localization.[2]
Structure and interactions
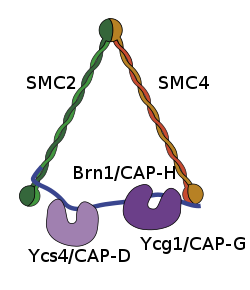
As one of the main subunits in the highly conserved SMC condensin I complex in eukaryotes, NCAPH associates with NCAPG, NCAPD2, and the N and C termini of the SMC-4 and SMC-2 proteins. NCAPH creates a bridge between the head groups of the SMC proteins and functions as a kleisin protein.[3][4][5]
The interaction between NCAPH and the globular ATPase head binding sites of the C terminus and N terminus of the SMC heterodimer allows condensin to have dynamic properties. The C terminus end of NCAPH assumes a winged-helix conformation, which then associates with either head group of the SMC protein. At the opposite end of the kleisin protein, the N terminus associates with proximal coiled coil of the other SMC protein, and creates a helical bundle.[4] This attribute enables the condensin complex to have open and closed conformations in order to associate with chromatin and aid in proper folding of DNA in the condensation process.[5][6]
Studies suggest that the sub-complex formed between NCAPH and NCAPG is critical for interactions with single-stranded DNA and double-stranded DNA to assist mitotic chromosome assembly in eukaryotes.[5]
Clinical significance
NCAPH may be used as a prognostic indicator of carcinogenesis in humans, as the abnormal over-expression of NCAPH is observed in many cancer types.[7]
Studies show that, in prostate cancer,[8] nasopharyngeal carcinoma,[9] hepatocellular carcinoma,[10] and breast cancers,[11] NCAPH is commonly over-expressed, and may be used as a biomarker for various cancer types and a viable prognostic factor for identification and potential drug targeting.[8]
In colon cancer, NCAPH is shown to be higher expressed in cancerous cells compared to non-cancerous epithelial cells. supplementally, when NCAPH is depleted, studies show a decrease in colon cancer cell proliferation.[7][12] Studies show that high expression of NCAPH in colon cancer and non-small cell lung cancer patients had an increased survival rate than those with a lower expression of NCAPH.[12]
References
- ↑ "Localization of BRRN1, the human homologue of Drosophila barr, to 2q11.2". Genomics 46 (2): 311–313. December 1997. doi:10.1006/geno.1997.5021. PMID 9417923.
- ↑ 2.0 2.1 "Entrez Gene: NCAPH non-SMC condensin I complex, subunit H". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=23397.
- ↑ 3.0 3.1 3.2 "Overexpression of NCAPH is upregulated and predicts a poor prognosis in prostate cancer". Oncology Letters 17 (6): 5768–5776. June 2019. doi:10.3892/ol.2019.10260. PMID 31186803.
- ↑ 4.0 4.1 "Kite Proteins: a Superfamily of SMC/Kleisin Partners Conserved Across Bacteria, Archaea, and Eukaryotes". Structure 23 (12): 2183–2190. December 2015. doi:10.1016/j.str.2015.10.004. PMID 26585514.
- ↑ 5.0 5.1 5.2 "Structural basis of HEAT-kleisin interactions in the human condensin I subcomplex". EMBO Reports 20 (5). May 2019. doi:10.15252/embr.201847183. PMID 30858338.
- ↑ "Kite Proteins: a Superfamily of SMC/Kleisin Partners Conserved Across Bacteria, Archaea, and Eukaryotes". Structure 23 (12): 2183–2190. December 2015. doi:10.1016/j.str.2015.10.004. PMID 26585514.
- ↑ 7.0 7.1 "NCAPH plays important roles in human colon cancer". Cell Death & Disease 8 (3): e2680. March 2017. doi:10.1038/cddis.2017.88. PMID 28300828.
- ↑ 8.0 8.1 "Overexpression of NCAPH is upregulated and predicts a poor prognosis in prostate cancer". Oncology Letters 17 (6): 5768–5776. June 2019. doi:10.3892/ol.2019.10260. PMID 31186803.
- ↑ "Aberrant expression of β-catenin and E-cadherin is correlated with poor prognosis of nasopharyngeal cancer". Human Pathology 44 (7): 1357–1364. July 2013. doi:10.1016/j.humpath.2012.10.025. PMID 23375645.
- ↑ "Non-SMC condensin I complex subunit H enhances proliferation, migration, and invasion of hepatocellular carcinoma". Molecular Carcinogenesis 58 (12): 2266–2275. December 2019. doi:10.1002/mc.23114. PMID 31523845.
- ↑ "Identification of NCAPH as a biomarker for prognosis of breast cancer". Molecular Biology Reports 47 (10): 7831–7842. October 2020. doi:10.1007/s11033-020-05859-9. PMID 33009967.
- ↑ 12.0 12.1 "NCAPH is negatively associated with Mcl‑1 in non‑small cell lung cancer". Molecular Medicine Reports 22 (4): 2916–2924. October 2020. doi:10.3892/mmr.2020.11359. PMID 32945371.
Further reading
- "Prediction of the coding sequences of unidentified human genes. II. The coding sequences of 40 new genes (KIAA0041-KIAA0080) deduced by analysis of cDNA clones from human cell line KG-1". DNA Research 1 (5): 223–229. 1995. doi:10.1093/dnares/1.5.223. PMID 7584044.
- "Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein". Cell 89 (4): 511–521. May 1997. doi:10.1016/S0092-8674(00)80233-0. PMID 9160743.
- "Chromosome condensation by a human condensin complex in Xenopus egg extracts". The Journal of Biological Chemistry 276 (8): 5417–5420. February 2001. doi:10.1074/jbc.C000873200. PMID 11136719.
- "Cell cycle-dependent expression and nucleolar localization of hCAP-H". Molecular Biology of the Cell 12 (11): 3527–3537. November 2001. doi:10.1091/mbc.12.11.3527. PMID 11694586.
- "Condensin I interacts with the PARP-1-XRCC1 complex and functions in DNA single-strand break repair". Molecular Cell 21 (6): 837–848. March 2006. doi:10.1016/j.molcel.2006.01.036. PMID 16543152.
- "Phosphoproteome analysis of the human mitotic spindle". Proceedings of the National Academy of Sciences of the United States of America 103 (14): 5391–5396. April 2006. doi:10.1073/pnas.0507066103. PMID 16565220. Bibcode: 2006PNAS..103.5391N.
- "A probability-based approach for high-throughput protein phosphorylation analysis and site localization". Nature Biotechnology 24 (10): 1285–1292. October 2006. doi:10.1038/nbt1240. PMID 16964243.
 |
