Biology:Nematode Her-1
From HandWiki
| Caenor_Her-1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
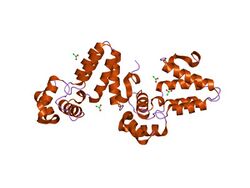 crystal structure of c. elegans her-1 | |||||||||
| Identifiers | |||||||||
| Symbol | Caenor_Her-1 | ||||||||
| Pfam | PF09232 | ||||||||
| InterPro | IPR015313 | ||||||||
| SCOP2 | 1szh / SCOPe / SUPFAM | ||||||||
| |||||||||
In molecular biology the nematode Her-1 protein is a protein which adopts an all-helical structure with two subdomains: amino acids 19-80 comprise a left-handed three-helix bundle with an overhand connection between the second and third helices, whilst amino acids 81-164 comprise a left-handed anti-parallel four-helix bundle in which the first helix consists of four consecutive turns of 3-10-helix. Fourteen cysteines are conserved in all known HER-1 sequences and form seven disulphide bonds. The protein dictates male development in Caenorhabditis elegans, probably by playing a direct role in cell signalling during C. elegans sex determination. It also inhibits the function of tra-2a.[1]
References
- ↑ "Crystal structure of Caenorhabditis elegans HER-1 and characterization of the interaction between HER-1 and TRA-2A". Proc. Natl. Acad. Sci. U.S.A. 101 (32): 11673–8. August 2004. doi:10.1073/pnas.0402559101. PMID 15289613.
 |

