Biology:Olinciguat
From HandWiki
Short description: Chemical compound
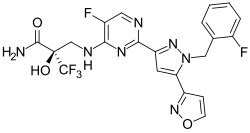 | |
| Clinical data | |
|---|---|
| Other names | IW-1701 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H16F5N7O3 |
| Molar mass | 509.397 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Olinciguat (IW-1701) is a soluble guanylate cyclase stimulator that was in development for sickle cell anemia.[1][2][3] After receiving orphan drug status in 2018[4] and completing a phase II trial, its development for sickle cell anemia was discontinued in 2020.[5]
References
- ↑ Buys, E. S.; Zimmer, D. P.; Chickering, J.; Graul, R.; Chien, Y. T.; Profy, A.; Hadcock, J. R.; Masferrer, J. L. et al. (1 August 2018). "Discovery and development of next generation sGC stimulators with diverse multidimensional pharmacology and broad therapeutic potential". Nitric Oxide 78: 72–80. doi:10.1016/j.niox.2018.05.009. ISSN 1089-8603. PMID 29859918. https://www.sciencedirect.com/science/article/pii/S1089860318300715.
- ↑ Tchernychev, Boris; Li, Huihui; Lee, Sung-Kyun; Gao, Xin; Ramanarasimhaiah, Raghunath; Liu, Guang; Hall, Katherine C.; Bernier, Sylvie G. et al. (September 2021). "Olinciguat, a stimulator of soluble guanylyl cyclase, attenuates inflammation, vaso-occlusion and nephropathy in mouse models of sickle cell disease". British Journal of Pharmacology 178 (17): 3463–3475. doi:10.1111/bph.15492. ISSN 1476-5381. PMID 33864386.
- ↑ Zimmer, Daniel P.; Shea, Courtney M.; Tobin, Jenny V.; Tchernychev, Boris; Germano, Peter; Sykes, Kristie; Banijamali, Ali R.; Jacobson, Sarah et al. (8 April 2020). "Olinciguat, an Oral sGC Stimulator, Exhibits Diverse Pharmacology Across Preclinical Models of Cardiovascular, Metabolic, Renal, and Inflammatory Disease". Frontiers in Pharmacology 11: 419. doi:10.3389/fphar.2020.00419. ISSN 1663-9812. PMID 32322204.
- ↑ "Ironwood Pharmaceuticals Announces FDA Orphan Drug Designation for Olinciguat for the Treatment of Sickle Cell Disease". https://investor.ironwoodpharma.com/press-releases/press-release-details/2018/Ironwood-Pharmaceuticals-Announces-FDA-Orphan-Drug-Designation-for-Olinciguat-for-the-Treatment-of-Sickle-Cell-Disease/default.aspx.
- ↑ PhD, Joana Carvalho (20 October 2020). "Cyclerion Suspends Development of Olinciguat for Sickle Cell Disease". https://sicklecellanemianews.com/news/cyclerion-suspends-development-of-olinciguat-for-sickle-cell-disease/.
 |

