Biology:Phototrexate
From HandWiki
Short description: Photopharmacological agent
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
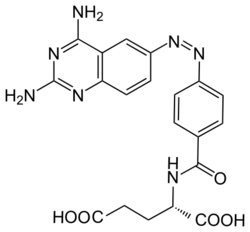
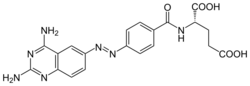
| Formula | C20H19N7O5 |
| Molar mass | 437.416 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Phototrexate is a photochromic antifolate drug developed at the Institute for Bioengineering of Catalonia (IBEC, The Barcelona Institute of Science and Technology). In particular, it is a photopharmacological agent[1][2] that behaves as light-regulated inhibitor of the dihydrofolate reductase (DHFR) enzyme.[3][4] Phototrexate is a photoisomerizable structural analogue of the chemotherapy agent methotrexate. It is also an example of "azologization".[5] Pharmacological effects of phototrexate can be switched on and off by UVA and visible light, respectively. Phototrexate is almost inactive in its trans configuration while it behaves as a potent antifolate in its cis configuration. It can also spontaneously self-deactivate in the dark.
See also
References
- ↑ "Photopharmacology: beyond proof of principle". Journal of the American Chemical Society 136 (6): 2178–91. February 2014. doi:10.1021/ja413063e. PMID 24456115. https://www.rug.nl/research/portal/en/publications/photopharmacology(d6714f52-c2c8-4e48-b345-238e98bcc776).html.
- ↑ "A roadmap to success in photopharmacology". Accounts of Chemical Research 48 (7): 1947–60. July 2015. doi:10.1021/acs.accounts.5b00129. PMID 26103428.
- ↑ "Photoswitchable Antimetabolite for Targeted Photoactivated Chemotherapy". Journal of the American Chemical Society 140 (46): 15764–15773. November 2018. doi:10.1021/jacs.8b08249. PMID 30346152.
- ↑ "Light-Wavelength-Based Quantitative Control of Dihydrofolate Reductase Activity by Using a Photochromic Isostere of an Inhibitor". ChemBioChem 20 (11): 1382–1386. June 2019. doi:10.1002/cbic.201800816. PMID 30656808.
- ↑ "Development of a new photochromic ion channel blocker via azologization of fomocaine". ACS Chemical Neuroscience 5 (7): 514–8. July 2014. doi:10.1021/cn500070w. PMID 24856540.
External links
 |