Biology:RoGFP
| reduction-oxidation sensitive Green fluorescent protein (roGFP) | |
|---|---|
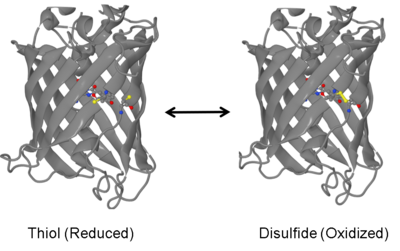 The oxidized and reduced forms of the redox-sensitive Green Fluorescent Protein 1-R7 (roGFP1-R7).[1] | |
| Identifiers | |
| Symbol | roGFP |
| PDB | 1JC1 |
The reduction-oxidation sensitive green fluorescent protein (roGFP) is a green fluorescent protein engineered to be sensitive to changes in the local redox environment. roGFPs are used as redox-sensitive biosensors.
In 2004, researchers in S. James Remington's lab at the University of Oregon constructed the first roGFPs by introducing two cysteines into the beta barrel structure of GFP. The resulting engineered protein could exist in two different oxidation states (reduced dithiol or oxidized disulfide), each with different fluorescent properties.[2]
Originally, members of the Remington lab published six versions of roGFP, termed roGFP1-6 (see more structural details below). Different groups of researchers introduced cysteines at different locations in the GFP molecule, generally finding that cysteines introduced at the amino acid positions 147 and 204 produced the most robust results.[3]
roGFPs are often genetically encoded into cells for in-vivo imaging of redox potential. In cells, roGFPs can generally be modified by redox enzymes such as glutaredoxin or thioredoxin. roGFP2 preferentially interacts with glutaredoxins and therefore reports the cellular glutathione redox potential.[4]
Various attempts have been made to make roGFPs that are more amenable to live-cell imaging. Most notably, substituting three positively-charged amino acids adjacent to the disulfide in roGFP1 drastically improves the response rate of roGFPs to physiologically relevant changes in redox potential. The resulting roGFP variants, named roGFP1-R1 through roGFP1-R14, are much more suitable for live-cell imaging.[1] The roGFP1-R12 variant has been used to monitor redox potential in bacteria and yeast,[5][6] but also for studies of spatially-organized redox potential in live, multicellular organisms such as the model nematode C. elegans.[7] In addition, roGFPs are used to investigate the topology of ER proteins, or to analyze the ROS production capacity of chemicals.[8] [9]
One notable improvement to roGFPs occurred in 2008, when the specificity of roGFP2 for glutathione was further increased by linking it to the human glutaredoxin 1 (Grx1).[10] By expressing the Grx1-roGFP fusion sensors in the organism of interest and/or targeting the protein to a cellular compartment, it is possible to measure the glutathione redox potential in a specific cellular compartment in real-time and therefore provides major advantages compared to other invasive static methods e.g. HPLC.
Given the variety of roGFPs, some effort has been made to benchmark their performance. For example, members of Javier Apfeld's group published a method in 2020 describing the 'suitable ranges' of different roGFPs, determined by how sensitive each sensor is to experimental noise in different redox conditions.[11]
Species of roGFP
See Kostyulk 2020 [12] for a more comprehensive review of different redox sensors.
| Name | Analyte | Citation |
|---|---|---|
| roGFP1-roGFP6 | EGSH | [2] |
| roGFP1_Rx Family | EGSH | [1] |
| roGFP1-iX Family | EGSH | [13] |
| Grx1-roGFP2 | EGSH | [10] |
| Mrx1-roGFP2 | EMSH | [14] |
| Brx-roGFP2 | EBSH | [15] |
| Tpx-roGFP2 | ET(SH)2 | [16] |
| Orp1-roGFP2 | H2O2 | [17] |
| roGFP2-Tsa2DCR | H2O2 | [18] |
See also
References
- ↑ 1.0 1.1 1.2 "Re-engineering redox-sensitive green fluorescent protein for improved response rate". Protein Science 15 (1): 45–57. January 2006. doi:10.1110/ps.051734306. PMID 16322566.
- ↑ 2.0 2.1 "Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators". The Journal of Biological Chemistry 279 (13): 13044–53. March 2004. doi:10.1074/jbc.M312846200. PMID 14722062.
- ↑ "Confocal imaging of glutathione redox potential in living plant cells". Journal of Microscopy 231 (2): 299–316. August 2008. doi:10.1111/j.1365-2818.2008.02030.x. PMID 18778428.
- ↑ "Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer". The Plant Journal 52 (5): 973–86. December 2007. doi:10.1111/j.1365-313X.2007.03280.x. PMID 17892447.
- ↑ "Harnessing the respiration machinery for high-yield production of chemicals in metabolically engineered Lactococcus lactis". Metabolic Engineering 44: 22–29. November 2017. doi:10.1016/j.ymben.2017.09.001. PMID 28890188. https://backend.orbit.dtu.dk/ws/files/136922112/1_s2.0_S1096717617301544_main.pdf.
- ↑ "Monitoring oxidative stress and DNA damage induced by heavy metals in yeast expressing a redox-sensitive green fluorescent protein". Current Microbiology 58 (5): 504–10. May 2009. doi:10.1007/s00284-008-9354-y. PMID 19184609.
- ↑ "Regulated spatial organization and sensitivity of cytosolic protein oxidation in Caenorhabditis elegans". Nature Communications 5 (1): 5020. September 2014. doi:10.1038/ncomms6020. PMID 25262602. Bibcode: 2014NatCo...5.5020R.
- ↑ "Non-invasive topology analysis of membrane proteins in the secretory pathway". The Plant Journal 57 (3): 534–41. February 2009. doi:10.1111/j.1365-313X.2008.03704.x. PMID 18939964.
- ↑ "Monitoring the in vivo redox state of plant mitochondria: effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge". Biochimica et Biophysica Acta (BBA) - Bioenergetics 1787 (5): 468–75. May 2009. doi:10.1016/j.bbabio.2009.01.020. PMID 19366606.
- ↑ 10.0 10.1 "Real-time imaging of the intracellular glutathione redox potential". Nature Methods 5 (6): 553–9. June 2008. doi:10.1038/NMETH.1212. PMID 18469822.
- ↑ "The SensorOverlord predicts the accuracy of measurements with ratiometric biosensors". Scientific Reports 10 (1): 16843. October 2020. doi:10.1038/s41598-020-73987-0. PMID 33033364. Bibcode: 2020NatSR..1016843S.
- ↑ "In Vivo Imaging with Genetically Encoded Redox Biosensors". International Journal of Molecular Sciences 21 (21): 8164. October 2020. doi:10.3390/ijms21218164. PMID 33142884.
- ↑ "Development of a family of redox-sensitive green fluorescent protein indicators for use in relatively oxidizing subcellular environments". Biochemistry 47 (33): 8678–88. August 2008. doi:10.1021/bi800498g. PMID 18652491.
- ↑ . Christopher M. Sassetti (ed.)"Reengineering redox sensitive GFP to measure mycothiol redox potential of Mycobacterium tuberculosis during infection". PLOS Pathogens 10 (1): e1003902. January 2014. doi:10.1371/journal.ppat.1003902. PMID 24497832.
- ↑ "Real-Time Imaging of the Bacillithiol Redox Potential in the Human Pathogen Staphylococcus aureus Using a Genetically Encoded Bacilliredoxin-Fused Redox Biosensor". Antioxidants & Redox Signaling 26 (15): 835–848. May 2017. doi:10.1089/ars.2016.6733. PMID 27462976.
- ↑ "A tryparedoxin-coupled biosensor reveals a mitochondrial trypanothione metabolism in trypanosomes". eLife 9: –53227. January 2020. doi:10.7554/eLife.53227. PMID 32003744.
- ↑ "Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases". The Journal of Biological Chemistry 284 (46): 31532–40. November 2009. doi:10.1074/jbc.M109.059246. PMID 19755417.
- ↑ "Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes". Nature Chemical Biology 12 (6): 437–43. June 2016. doi:10.1038/nchembio.2067. PMID 27089028.
 |
