Biology:Stolon


In biology, stolons (from Latin stolō, genitive stolōnis – "branch"), also known as runners, are horizontal connections between parts of an organism. They may be part of the organism, or of its skeleton. Typically, animal stolons are exoskeletons (external skeletons).
In botany
In botany, stolons are plant stems which grow at the soil surface or just below ground that form adventitious roots at the nodes, and new plants from the buds.[1][2] Stolons are often called runners. Rhizomes, in contrast, are root-like stems that may either grow horizontally at the soil surface or in other orientations underground.[1] Thus, not all horizontal stems are called stolons. Plants with stolons are called stoloniferous.
A stolon is a plant propagation strategy and the complex of individuals formed by a mother plant and all its clones produced from stolons form a single genetic individual, a genet.[citation needed]
Morphology

Stolons may have long or short internodes. The leaves along the stolon are usually very small, but in a few cases such as Stachys sylvatica are normal in size.[3]
Stolons arise from the base of the plant.[4] In strawberries the base is above the soil surface; in many bulb-forming species and plants with rhizomes, the stolons remain underground and form shoots that rise to the surface at the ends or from the nodes. The nodes of the stolons produce roots, often all around the node and hormones produced by the roots cause the stolon to initiate shoots with normal leaves.[5] Typically after the formation of the new plant the stolon dies away[6] in a year or two, while rhizomes persist normally for many years or for the life of the plant, adding more length each year to the ends with active growth. The horizontal growth of stolons results from the interplay of different hormones produced at the growing point and hormones from the main plant, with some studies showing that stolon and rhizome growth are affected by the amount of shady light the plant receives with increased production and branching from plants exposed to mixed shade and sun, while plants in all day sun or all shade produce fewer stolons.[7]
A number of plants have soil-level or above-ground rhizomes, including Iris species and many orchid species.
T. Holm (1929) restricted the term rhizome to a horizontal, usually subterranean, stem that produces roots from its lower surface and green leaves from its apex, developed directly from the plumule of the embryo. He recognized stolons as axillary, subterranean branches that do not bear green leaves but only membranaceous, scale-like ones.[8]
A stolon of grasses is defined as a horizontal stem above or on the soil surface that often roots at the internodes.[9]
Plants with stolons

In some Cyperus species the stolons end with the growth of tubers; the tubers are swollen stolons that form new plants.[10]
Some species of crawling plants can also sprout adventitious roots, but are not considered stoloniferous: a stolon is sprouted from an existing stem and can produce a full individual. Examples of plants that extend through stolons include some species from the genera Riccia, Argentina (silverweed), Cynodon, Fragaria, and Pilosella (Hawkweeds), Zoysia japonica, Ranunculus repens. Plants with long, slender stolons are referred to as sarmentose plants.[11]
Other plants with stolons below the soil surface include many grasses, Ajuga, Mentha,[12] and Stachys. Several species of Irises have stolons attached to their rhizomes,[13] including Iris stolonifera.
Lily-of-the-valley (Convallaria majalis) has rhizomes that grow stolon-like stems called stoloniferous rhizomes or leptomorph rhizomes. A number of plants have stoloniferous rhizomes including Asters.[14] These stolon-like rhizomes are long and thin, with long internodes and indeterminate growth with lateral buds at the node, which mostly remain dormant.[citation needed]
In potatoes, the stolons[15] start to grow within 10 days of plants emerging above ground, with tubers usually beginning to form on the end of the stolons.[16] The tubers are modified stolons[17] that hold food reserves, with a few buds that grow into stems. Since it is not a rhizome it does not generate roots, but the new stem growth that grows to the surface produces roots. See also BBCH-scale (potato).
Hydrilla use stolons that produce tubers to spread themselves and to survive dry periods in aquatic habitats.[18]
Erythronium, commonly called Trout Lily, have white stolons growing from the bulb. Most run horizontally, either underground or along the surface of the ground under leaf litter. A number of bulbous species produce stolons, such as Erythronium propullans. Flowering plants often produce no stolons.[19]
Convolvulus arvensis is a weed species in agriculture that spreads by under ground stolons that produce rhizomes.[20]
In studies on grass species, with plants that produce stolons or rhizomes and plants that produce both stolons and rhizomes, morphological and physiological differences were noticed. Stolons have longer internodes and function as means of seeking out light and are used for propagation of the plant, while rhizomes are used as storage organs for carbohydrates and the maintenance of meristem tissue to keep the parent plant alive from one year to the next.[21]
In mycology
In mycology, a stolon is defined as an occasionally septate hypha, which connects sporangiophores together. Root-like structures called rhizoids may appear on the stolon as well, anchoring the hyphae to the substrate. The stolon is commonly found in bread molds, and are seen as horizontally expanding across the mold.
In zoology

Some bryozoans form colonies through connection of individual units by stolons. Other colonies include sheets and erect colonies.[22]
Some colonial Cnidaria develop as stolons with interconnected medusoid structures that later separate.[citation needed]
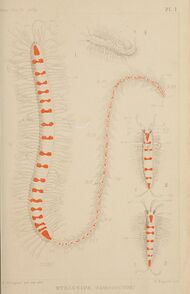
Some worm-like animals, such as certain Polychaeta in the genus Myrianida, form stolons containing eggs or sperm which trail behind the main body before detaching to mate with other stolons.[23]
The worm Megasyllis nipponica takes this to an extreme, developing stolons with their own eyes, antennae and brain which detach and mate with an opposite-sex stolon to produce fertilized eggs.[24]
In palaeontology
Stolon-based reproduction is thought to have been used by Rangeomorphs in the Ediacaran age.[25][26]
See also
| Wikimedia Commons has media related to Plant stolons. |
- Biology:Offshoot (plant) – Lateral growth branching from plant stem
- Biology:Root – Basal organ of a vascular plant
- Biology:Vegetative reproduction – Asexual method of reproduction in plants
References
- ↑ Jump up to: 1.0 1.1 Hickey, M.; King, C. (2001). The Cambridge Illustrated Glossary of Botanical Terms. Cambridge University Press.
- ↑ "Stolon". Dictionary.com. http://dictionary.reference.com/browse/stolon.
- ↑ Goebel, K. E. R. (1969). Organography of plants, especially of the Archegoniatae and Spermaphyta. New York: Hofner publishing company.
- ↑ Gleason, Henry A. (1963). The new Britton and Brown illustrated flora of the Northeastern United States and adjacent Canada, Volume 1. New York: Hafner Press. p. ixxiv. ISBN 0-02-845240-2.
- ↑ Woolley, D. J.; P. F. Wareing (March 1972). "The role of roots, cytokinins and apical dominance in the control of lateral shoot form in Solanum andigena". Planta 105 (1): 33–42. doi:10.1007/BF00385161. PMID 24477700.
- ↑ Wijesinghe, Dushyantha K.; Dennis F. Whigham (June 2001). "Nutrient foraging in woodland herbs: a comparison of three species of Uvularia (Liliaceae) with contrasting belowground morphologies". American Journal of Botany 88 (6): 1071–1079. doi:10.2307/2657090. PMID 11410472.
- ↑ Méthy, M.; P. Alpert; J. Roy (September 1990). "Effects of light quality and quantity on growth of the clonal plant Eichhornia crassipes". Oecologia 84 (2): 265–271. doi:10.1007/BF00318283. PMID 28312764. Bibcode: 1990Oecol..84..265M.
- ↑ Henderson, Norton C.. "Iris". Flora of North America. eFloras.org. http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=116503.
- ↑ Aldous, David E.; Chivers, Ian H. (2002-06-13). Sports Turf and Amenity Grasses: A Manual for Use and Identification. Landlinks Press. ISBN 978-0-643-09960-9. https://books.google.com/books?id=bfFuyRs_aMQC&pg=PP13.
- ↑ Tucker, Gordon C.; Marcks, Brian G.; Carter, J. R.. "Cyperus serotinus". Flora of North America. eFloras.org. http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=242101127.
- ↑ Chisholm, Hugh, ed (1911). "Sarmentose". Encyclopædia Britannica. 24 (11th ed.). Cambridge University Press. p. 220.
- ↑ Aflatuni, Abbas; Uusitalo, J.; Ek, S.; Hohtola, A. (February 2005). "Variation in the Amount of Yield and in the Extract Composition Between Conventionally Produced and Micropropagated Peppermint and Spearmint". Journal of Essential Oil Research 17 (1): 66–70. doi:10.1080/10412905.2005.9698833. ISSN 1041-2905.
- ↑ "Home". http://encyclopaedia.alpinegardensociety.net/plants/Iris.
- ↑ Jones, Almut G. (January 1978). "Observations on Reproduction and Phenology in Some Perennial Asters". American Midland Naturalist 99 (1): 184–97. doi:10.2307/2424942.
- ↑ Visser, Richard G. F.; Vreugdenhil, Dick; Hendriks, Theo; Evert Jacobsen (February 1994). "Gene expression and carbohydrate content during stolon to tuber transition in potatoes (Solanum tuberosum)". Physiologia Plantarum 90 (2): 285–92. doi:10.1111/j.1399-3054.1994.tb00389.x.
- ↑ Monaco Educational Service. "Introduction to stems". Botany. http://www.personal.psu.edu/faculty/w/x/wxm15/Online/Botany/Stems/stem_lecture_01.htm.
- ↑ Hartmann, Hudson Thomas; Dale E Kester (1983). Plant propagation : principles and practices. Englewood Cliffs: Prentice-Hall. p. 508. ISBN 0-13-681007-1.
- ↑ "Hydrilla in the Catawba River Basin". NCSU Aquatic Weed Management Program. http://www.weedscience.ncsu.edu/aquaticweeds/catawba.pdf.
- ↑ weakley, Alan S.. "Flora of the Carolinas, Virginia, and Georgia, and Surrounding Areas, Part 6". pp. 808. http://www.herbarium.unc.edu/WeakleysFloraPart6.pdf.
- ↑ Harris, Peter. "Field and Hedge bindweeds Convolvulus arvensis L. and Calystegia sepium (L.) R. Br.". Classical Biological Control of Weeds. Agriculture and Agri-Food Canada, Lethbridge Research Centre. http://res2.agr.ca/lethbridge/weedbio/plant/convolvulus_e.htm.
- ↑ Pierdominici, Maria Grazia; Ming Dong (January 1995). "Morphology and growth of stolons and rhizomes in three clonal grasses, as affected by different light supply". Plant Ecology 116 (1): 25–32. doi:10.1007/BF00045274. https://link.springer.com/article/10.1007/BF00045274.
- ↑ Levinton, Jeffrey S. "Marine Biology." 3rd Edition. Oxford Press. 2008.
- ↑ Pleijel, Fredrik (16 September 2016). "Runner up: Evolutionary Biology. “Polychaetous worm with engine and wagons”". Royal Society Photo Contest Winners Capture Breathtaking Details of our Rapidly Changing World. http://designyoutrust.com/2016/09/royal-society-photo-contest-winners-capture-breathtaking-details-of-our-rapidly-changing-world/. "This trainworm (Myrianida pinnigera), which is 35 mm from head to tail, lives on the sea floor. Its front end, the trainworm’s engine, is followed by a row of carriages called ‘stolons’ that increase in size towards the worm’s tail end. The carriages are the worm’s swimming sexual organs. When the trainworm is mature, the last carriage in the train lets go and detaches. It swims up the water column to reproduce. The carriages, unlike the engine, all lack a gut and are full of either sperm or eggs. In the water column the well-developed sensory organs seek out another stolon to mate with. After mating the male stolons die. The females survive a short time to shelter the embryos, which are carried in their bellies. Meanwhile, the trainworm on the sea floor continues to produce stolon carriages."
- ↑ Rayne, Elizabeth (8 December 2023). "Worm’s rear end develops its own head, wanders off to mate". Ars Technica. https://arstechnica.com/science/2023/12/bizarre-worm-can-detach-its-own-butt-and-make-it-swim-away/. "This appendage will detach from the main body and swim away, carrying gonads that will merge with those from other disembodied rear ends and give rise to a new generation. Wait, what in the science fiction B-movie alien star system is this thing?"
- ↑ Mitchell et al. (2015), doi:10.1038/nature14646
- ↑ Peterson et al. (2003), Integr Comp Biol, 43:127-36
 |