Biology:Tyrosine ammonia-lyase
From HandWiki
| Tyrosine ammonia lyase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 4.3.1.23 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Tyrosine ammonia lyase (EC 4.3.1.23, L-tyrosine ammonia-lyase, TAL or Tyrase) is an enzyme in the natural phenols biosynthesis pathway. It transforms L-tyrosine into p-coumaric acid.[1][2][3]
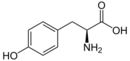 130px|para-coumaric acid + Ammonia + H+
130px|para-coumaric acid + Ammonia + H+
L-tyrosine = trans-p-hydroxycinnamate + NH3
See also
- EC 4.3.1.24 (phenylalanine ammonia-lyase)
- EC 4.3.1.25 (phenylalanine/tyrosine ammonia-lyase)
References
- ↑ "Structural determinants and modulation of substrate specificity in phenylalanine-tyrosine ammonia-lyases". Chemistry & Biology 13 (12): 1327–38. December 2006. doi:10.1016/j.chembiol.2006.11.011. PMID 17185228.
- ↑ "Discovery of a substrate selectivity switch in tyrosine ammonia-lyase, a member of the aromatic amino acid lyase family". Chemistry & Biology 13 (12): 1317–26. December 2006. doi:10.1016/j.chembiol.2006.10.008. PMID 17185227.
- ↑ "Crystal structure of histidine ammonia-lyase revealing a novel polypeptide modification as the catalytic electrophile". Biochemistry 38 (17): 5355–61. April 1999. doi:10.1021/bi982929q. PMID 10220322.
External links
- Tyrosine+ammonia-lyase at the US National Library of Medicine Medical Subject Headings (MeSH)
- www.hhmi.org
 |

