Biology:WNK1
 Generic protein structure example |
WNK (lysine deficient protein kinase 1), also known as WNK1, is an enzyme that is encoded by the WNK1 gene.[1][2][3][4][5] WNK1 is serine-threonine protein kinase and part of the "with no lysine/K" kinase WNK family.[1][2][3][5] The predominant role of WNK1 is the regulation of cation-Cl− cotransporters (CCCs) such as the sodium chloride cotransporter (NCC), basolateral Na-K-Cl symporter (NKCC1), and potassium chloride cotransporter (KCC1) located within the kidney.[1][2][5] CCCs mediate ion homeostasis and modulate blood pressure by transporting ions in and out of the cell.[1] WNK1 mutations as a result have been implicated in blood pressure disorders/diseases; a prime example being familial hyperkalemic hypertension (FHHt).[1][2][3][4][5]
Structure
The WNK1 protein is composed of 2382 amino acids (molecular weight 230 kDa).[4] The protein contains a kinase domain located within its short N-terminaldomain and a long C-terminal tail.[4] The kinase domain has some similarity to the MEKK protein kinase family.[4] As a member of the WNK family, the kinase's catalytic lysine residue is uniquely located in beta strand 2 of the glycine loop.[4] In order to have kinase activity, WNK1 must autophosphorylate the serine 382 residue found in its activation loop.[4][1] Further, phosphorylation at another site (Ser378) increases WNK1 activity.[1] An autoinhibitory domain is located within the C-terminal domain along with a HQ domain that is needed for WNK1 interactions with other WNKs.[1][2][3][4] The interactions between WNKs play an important role in function; WNK1 mutants that lack an HQ domain also lack kinase activity.
Function
The WNK1 gene encodes a cytoplasmic serine-threonine kinase expressed in the distal nephron.[1][2][4] Studies have shown that WNK1 can activate multiple CCCs.[1][2] WNK1 however, does not directly phosphorylate the CCCs themselves rather it phosphorylates other serine-threonine kinases: Sterile20 related proline-alanine-rich kinase (SPAK) and oxidative stress response kinase 1 (OXSR1).[2][1][3] Phosphorylation of SPAK's T loop located in its catalytic domain will activate SPAK, which will go on to phosphorylation the CCC's N-terminaldomain.[1][2] Hence, WNK1 activates CCCs indirectly as an upstream regulator of SPAK/OSR1.[1][2][3]
Sodium reabsorption

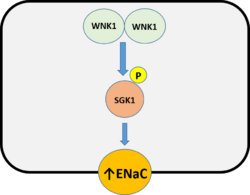
In the distal convoluted tubule (DCT), WNK1 is a potent activator of the NCC that results in an increase in sodium re absorption that drives an increase in blood pressure.[1][2][3] The WNK1 mutant found in FHHt harbors a large deletion within intron 1 that causes an increase in the expression of full length WNK1.[1][2][3][4] The boost in WNK1 leads to increases in NCC activation that promotes the high blood pressure/hypertension associated with FHHt.[1][2][3][4] WNK1 activates the serum-and glucocorticoid-inducible protein kinase SGK1, leading to increased expression of the epithelial sodium channel (ENaC), which also promotes sodium re absorption.[2]
Potassium secretion
WNK1 regulates potassium channels found in the cortical collecting duct (CCD) and connecting tubule (CNT).[2] Renal outer medullar potassium 1 (ROMK1) and large conductance calcium-activated potassium channel (BKCa) are the two primary channels for potassium secretion.[2] WNK1 indirectly stimulates clathrin-dependent endocytosis of ROMK1 by a potential interaction with intersectin (ITSN1); thus, kinase activity is not needed.[2] Another possible mechanism of ROMK1 regulation is via autosomal recessive hypercholesterolemia (ACH), which is a clathrin adaptor molecule.[2] ACH phosphorylation by WNK1 promotes the translocation of ROMK1 to clathrin coated pits triggering endocytosis.[2] WNK1 may indirectly activate BKCa by inhibiting the actions of extracellular signal–regulated kinases (ERK1 and ERK2) that lead to lysomal degradation.[2]
Cell volume regulation
The NKCC1/2 cotransporters are regulated by intracellular Cl− concentration.[5] Studies point to WNK1 as key effector that couples Cl− concentration to NKCC1/2 function.[1][5] In hypertonic (high extracellular Cl− ) conditions that trigger cell shrinkage, an unknown mechanism upregulates WNK1 expression to counteract the volume loss.[1] The increased WNK1 leads to activation of SPAK/OSR1 that activate NKCC1/2 via subsequent phosphorylation.[1][5] NKCC1/2 will promote the influx of Na+, K+, and Cl− ions into the cell thereby causing the flow of water into the cell.[1] In the reverse circumstances, where hypotonic (low extracellular Cl− ) conditions induce cell swelling, WNK1 is inhibited.[1] Another cotransporter, KCC is inactive when phosphorylated; without activated WNK1, KCC does not undergo phosphorylation and can activate.[1] The cotransporter will promote the efflux of K+ and Cl− ions and cause the flow of water out of the cell to combat swelling.[1]
WNK1 in the brain
In the mature brain, the GABA neurotransmitter represents the major inhibitory signal used in neuronal signaling.[1] GABA activates the GABAA receptor which is a Cl− ion channel.[1] Cl− ions will enter the neuron causing hyperpolarization and inhibition of signaling.[1] During brain development however, GABAA activation will allow Cl− ions to leave the neuron causing the neuron to depolarize.[1] Thus, GABA is an excitatory neurotransmitter during development.[1] WNK1 has been implicated in the developmental switch from excitatory to inhibitory GABA signaling via interaction with NKCC1 and KCCs.[1] WNK1 phosphorylates SPAK/OSR1 which then phosphorylates KCC2 inhibiting the flow of Cl− ions out of the cell during development.[1]
Regulation of WNK1
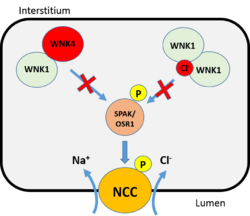
The concentrations of Cl− ions and K+ ion play a major role in regulating WNK1 activity.[1][5] In the DCT, the plasma concentration of K+ ion is thought to impact the concentration Cl− ions within the nephron.[1][5] High plasma K+ concentration down regulates WNK1 activity and prevents Cl− ion from entering the nephron via the NCC.[1][5] The opposite occurs when plasma K+ concentration is low; increased WNK1 activity boosts NCC activity promoting reabsorption of Cl− ions.[1][5] When there is an abundance of Cl− ions within the nephron, WNK1 activity is inhibited by the binding of a Cl− ion to WNK1's catalytic domain.[1][5]
Furthermore, WNK1 and WNK4 may interact to form heterodimers that inhibit WNK1 function.[3][2] WNK4 release from the heterodimer allows WNK1 monomer to bind another WNK1 monomer to promote activation.[2][3] WNK1 function can also be inhibited if WNK1 is degraded. There are two enzymes responsible for WNK1 ubiquitination, kelch like 3 (KLHL3) and cullin 3 (CUL3).[3][2][6] KLHL3 serves as an adaptor protein that promotes the interaction between WNK1 and Cullin3, which is in a complex containing an E3 ubiquitin ligase that attaches the ubiquitin molecules to WNK1.[3] The ubiquitinated WNK1 will subsequently undergo proteasomal degradation.[3][2][6]
Clinical significance
WNK1 has mutations associated with Gordon hyperkalemia-hypertension syndrome (pseudohypoaldosteronism Type II, featuring hypertension also called familial hyperkalemic hypertension (FHHt) )[1][3][4] and congenital sensory neuropathy (HSAN Type II, featuring loss of perception to pain, touch, and heat due to a loss of peripheral sensory nerves).[1][7]
Comparative genomics
The gene belongs to a group of four related protein kinases (WNK1, WNK2, WNK3, WNK4).[1][3][4]
Homologs of this protein have been found in Arabidopsis thaliana, C. elegans, Chlamydomonas reinhardtii and Vitis viniferaas well as in vertebrates including Danio rerio and Taeniopygia guttata.[3]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 1.33 1.34 1.35 1.36 1.37 "WNK Kinase Signaling in Ion Homeostasis and Human Disease". Cell Metabolism 25 (2): 285–299. February 2017. doi:10.1016/j.cmet.2017.01.007. PMID 28178566.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 "Regulation of Renal Electrolyte Transport by WNK and SPAK-OSR1 Kinases". Annual Review of Physiology 78: 367–89. 2016. doi:10.1146/annurev-physiol-021115-105431. PMID 26863326.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 "Revisiting the NaCl cotransporter regulation by with-no-lysine kinases". American Journal of Physiology. Cell Physiology 308 (10): C779-91. May 2015. doi:10.1152/ajpcell.00065.2015. PMID 25788573.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 "WNK1: analysis of protein kinase structure, downstream targets, and potential roles in hypertension". Cell Research 15 (1): 6–10. January 2005. doi:10.1038/sj.cr.7290256. PMID 15686619.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 "A unifying mechanism for WNK kinase regulation of sodium-chloride cotransporter". Pflügers Archiv 467 (11): 2235–41. November 2015. doi:10.1007/s00424-015-1708-2. PMID 25904388.
- ↑ 6.0 6.1 "The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters". Science Signaling 7 (334): re3. July 2014. doi:10.1126/scisignal.2005365. PMID 25028718.
- ↑ "(WNK)ing at death: With-no-lysine (Wnk) kinases in neuropathies and neuronal survival" (in en). Brain Research Bulletin 125: 92–8. July 2016. doi:10.1016/j.brainresbull.2016.04.017. PMID 27131446.
External links
- Overview of all the structural information available in the PDB for UniProt: Q9H4A3 (Serine/threonine-protein kinase WNK1) at the PDBe-KB.
 |
