Biology:GABAA receptor
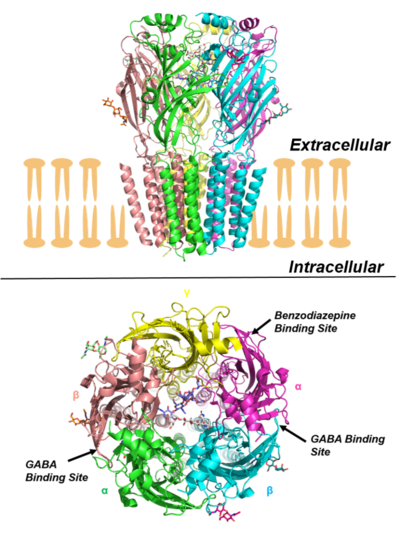
The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Accurate regulation of GABAergic transmission through appropriate developmental processes, specificity to neural cell types, and responsiveness to activity is crucial for the proper functioning of nearly all aspects of the central nervous system (CNS).[1] Upon opening, the GABAA receptor on the postsynaptic cell is selectively permeable to chloride ions (Cl−) and, to a lesser extent, bicarbonate ions (HCO3−).[2][3]
GABAAR are members of the ligand-gated ion channel receptor superfamily, which is a chloride channel family with a dozen or more heterotetrametric subtypes and 19 distinct subunits. These subtypes have distinct brain regional and subcellular localization, age-dependent expression, and the ability to undergo plastic alterations in response to experience, including drug exposure.[4]
GABAAR is not just the target of agonist depressants and antagonist convulsants, but most GABAAR medicines also act at additional (allosteric) binding sites on GABAAR proteins. Some sedatives and anxiolytics, such as benzodiazepines and related medicines, act on GABAAR subtype-dependent extracellular domain sites. Alcohols and neurosteroids, among other general anesthetics, act at GABAAR subunit-interface transmembrane locations. High anesthetic dosages of ethanol act on GABAAR subtype-dependent transmembrane domain locations. Ethanol acts at GABAAR subtype-dependent extracellular domain locations at low intoxication concentrations. Thus, GABAAR subtypes have pharmacologically distinct receptor binding sites for a diverse range of therapeutically significant neuropharmacological drugs.[5]
Depending on the membrane potential and the ionic concentration difference, this can result in ionic fluxes across the pore. If the membrane potential is higher than the equilibrium potential (also known as the reversal potential) for chloride ions, when the receptor is activated Cl− will flow into the cell.[6] This causes an inhibitory effect on neurotransmission by diminishing the chance of a successful action potential occurring at the postsynaptic cell. The reversal potential of the GABAA-mediated inhibitory postsynaptic potential (IPSP) in normal solution is −70 mV, contrasting the GABAB IPSP (-100 mV).
The active site of the GABAA receptor is the binding site for GABA and several drugs such as muscimol, gaboxadol, and bicuculline.[7] The protein also contains a number of different allosteric binding sites which modulate the activity of the receptor indirectly. These allosteric sites are the targets of various other drugs, including the benzodiazepines, nonbenzodiazepines, neuroactive steroids, barbiturates, alcohol (ethanol),[8] inhaled anaesthetics, kavalactones, cicutoxin, and picrotoxin, among others.[9]
Much like the GABAA receptor, the GABAB receptor is an obligatory heterodimer consisting of GABAB1 and GABAB2 subunits. These subunits include an extracellular Venus Flytrap domain (VFT) and a transmembrane domain containing seven α-helices (7TM domain). These structural components play a vital role in intricately modulating neurotransmission and interactions with drugs. [10]
Target for benzodiazepines
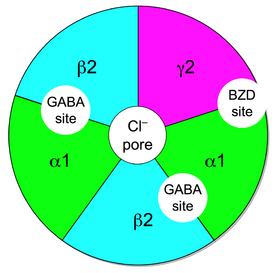
The ionotropic GABAA receptor protein complex is also the molecular target of the benzodiazepine class of tranquilizer drugs. Benzodiazepines do not bind to the same receptor site on the protein complex as does the endogenous ligand GABA (whose binding site is located between α- and β-subunits), but bind to distinct benzodiazepine binding sites situated at the interface between the α- and γ-subunits of α- and γ-subunit containing GABAA receptors.[11][12] While the majority of GABAA receptors (those containing α1-, α2-, α3-, or α5-subunits) are benzodiazepine sensitive, there exists a minority of GABAA receptors (α4- or α6-subunit containing) which are insensitive to classical 1,4-benzodiazepines,[13] but instead are sensitive to other classes of GABAergic drugs such as neurosteroids and alcohol. In addition peripheral benzodiazepine receptors exist which are not associated with GABAA receptors. As a result, the IUPHAR has recommended that the terms "BZ receptor", "GABA/BZ receptor" and "omega receptor" no longer be used and that the term "benzodiazepine receptor" be replaced with "benzodiazepine site".[14] Benzodiazepines like diazepam and midazolam act as positive allosteric modulators for GABAA receptors. When these receptors are activated, there's a rise in intracellular chloride levels, resulting in cell membrane hyperpolarization and decreased excitation.[15]
In order for GABAA receptors to be sensitive to the action of benzodiazepines they need to contain an α and a γ subunit, between which the benzodiazepine binds. Once bound, the benzodiazepine locks the GABAA receptor into a conformation where the neurotransmitter GABA has much higher affinity for the GABAA receptor, increasing the frequency of opening of the associated chloride ion channel and hyperpolarising the membrane. This potentiates the inhibitory effect of the available GABA leading to sedative and anxiolytic effects.[16]
Different benzodiazepines have different affinities for GABAA receptors made up of different collection of subunits, and this means that their pharmacological profile varies with subtype selectivity. For instance, benzodiazepine receptor ligands with high activity at the α1 and/or α5 tend to be more associated with sedation, ataxia and amnesia, whereas those with higher activity at GABAA receptors containing α2 and/or α3 subunits generally have greater anxiolytic activity.[17] Anticonvulsant effects can be produced by agonists acting at any of the GABAA subtypes, but current research in this area is focused mainly on producing α2-selective agonists as anticonvulsants which lack the side effects of older drugs such as sedation and amnesia.
The binding site for benzodiazepines is distinct from the binding site for barbiturates and GABA on the GABAA receptor, and also produces different effects on binding,[18] with the benzodiazepines increasing the frequency of the chloride channel opening, while barbiturates increase the duration of chloride channel opening when GABA is bound.[19] Since these are separate modulatory effects, they can both take place at the same time, and so the combination of benzodiazepines with barbiturates is strongly synergistic, and can be dangerous if dosage is not strictly controlled.[20]
Also note that some GABAA agonists such as muscimol and gaboxadol do bind to the same site on the GABAA receptor complex as GABA itself, and consequently produce effects which are similar but not identical to those of positive allosteric modulators like benzodiazepines.
Structure and function
Structural understanding of the GABAA receptor was initially based on homology models, obtained using crystal structures of homologous proteins like Acetylcholine binding protein (AChBP) and nicotinic acetylcholine (nACh) receptors as templates.[22][23][24] The much sought structure of a GABAA receptor was finally resolved, with the disclosure of the crystal structure of human β3 homopentameric GABAA receptor.[25] Whilst this was a major development, the majority of GABAA receptors are heteromeric and the structure did not provide any details of the benzodiazepine binding site. This was finally elucidated in 2018 by the publication of a high resolution cryo-EM structure of rat α1β1γ2S receptor[26] and human α1β2γ2 receptor bound with GABA and the neutral benzodiazepine flumazenil.[27]
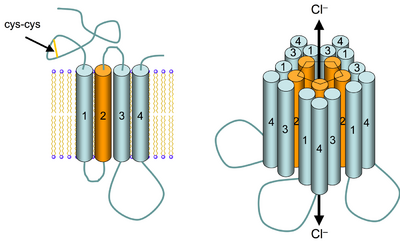
GABAA receptors are pentameric transmembrane receptors which consist of five subunits arranged around a central pore. Each subunit comprises four transmembrane domains with both the N- and C-terminus located extracellularly. The receptor sits in the membrane of its neuron, usually localized at a synapse, postsynaptically. However, some isoforms may be found extrasynaptically.[28] When vesicles of GABA are released presynaptically and activate the GABA receptors at the synapse, this is known as phasic inhibition. However, the GABA escaping from the synaptic cleft can activate receptors on presynaptic terminals or at neighbouring synapses on the same or adjacent neurons (a phenomenon termed ‘spillover’) in addition to the constant, low GABA concentrations in the extracellular space results in persistent activation of the GABAA receptors known as tonic inhibition.[29]
The ligand GABA is the endogenous compound that causes this receptor to open; once bound to GABA, the protein receptor changes conformation within the membrane, opening the pore in order to allow chloride anions (Cl−) and, to a lesser extent, bicarbonate ions (HCO3−) to pass down their electrochemical gradient. The binding site to GABA is about 80Å away from the narrowest part of the ion channel. Recent computational studies have suggested an allosteric mechanism whereby GABA binding leads to ion channel opening.[30] Because the reversal potential for chloride in most mature neurons is close to or more negative than the resting membrane potential, activation of GABAA receptors tends to stabilize or hyperpolarise the resting potential, and can make it more difficult for excitatory neurotransmitters to depolarize the neuron and generate an action potential. The net effect therefore typically inhibitory, reducing the activity of the neuron, although depolarizing currents have been observed in response to GABA in immature neurons in early development. This effect during development is due to a modified Cl− gradient wherein the anions leave the cells through the GABAA receptors, since their intracellular chlorine concentration is higher than the extracellular.[31] The difference in extracellular chlorine anion concentration is presumed to be due to the higher activity of chloride transporters, such as NKCC1, transporting chloride into cells which are present early in development, whereas, for instance, KCC2 transports chloride out of cells and is the dominant factor in establishing the chloride gradient later in development. These depolarization events have shown to be key in neuronal development.[32] In the mature neuron, the GABAA channel opens quickly and thus contributes to the early part of the inhibitory post-synaptic potential (IPSP).[33][34] The endogenous ligand that binds to the benzodiazepine site is inosine.[35]
Proper developmental, neuronal cell-type-specific, and activity-dependent GABAergic transmission control is required for nearly all aspects of CNS function.[36]
It has been proposed that the GABAergic system is disrupted in numerous neurodevelopmental diseases, including fragile X syndrome, Rett syndrome, and Dravet syndrome, and that it is a crucial potential target for therapeutic intervention.[37]
Subunits
GABAA receptors are members of the large pentameric ligand gated ion channel (previously referred to as "Cys-loop" receptors) super-family of evolutionarily related and structurally similar ligand-gated ion channels that also includes nicotinic acetylcholine receptors, glycine receptors, and the 5HT3 receptor. There are numerous subunit isoforms for the GABAA receptor, which determine the receptor's agonist affinity, chance of opening, conductance, and other properties.[38]
In humans, the units are as follows:
- six types of α subunits (GABRA1, GABRA2, GABRA3, GABRA4, GABRA5, GABRA6)
- three βs (GABRB1, GABRB2, GABRB3)
- three γs (GABRG1, GABRG2, GABRG3)
- as well as a δ (GABRD), an ε (GABRE), a π (GABRP), and a θ (GABRQ)
There are three ρ units (GABRR1, GABRR2, GABRR3); however, these do not coassemble with the classical GABAA units listed above,[39] but rather homooligomerize to form GABAA-ρ receptors (formerly classified as GABAC receptors but now this nomenclature has been deprecated[40]).
Combinatorial Arrays
Given the large number of GABAA receptors, a great diversity of final pentameric receptor subtypes is possible. Methods to produce cell-based laboratory access to a greater number of possible GABAA receptor subunit combinations allow teasing apart of the contribution of specific receptor subtypes and their physiological and pathophysiological function and role in the CNS and in disease.[41]
Distribution
GABAA receptors are responsible for most of the physiological activities of GABA in the central nervous system, and the receptor subtypes vary significantly. Subunit composition can vary widely between regions and subtypes may be associated with specific functions. The minimal requirement to produce a GABA-gated ion channel is the inclusion of an α and a β subunit.[42] The most common GABAA receptor is a pentamer comprising two α's, two β's, and a γ (α2β2γ). In neurons themselves, the type of GABAA receptor subunits and their densities can vary between cell bodies and dendrites.[43] Benzodiazepines and barbiturates amplify the inhibitory effects mediated by the GABAA receptor.[44] GABAA receptors can also be found in other tissues, including leydig cells, placenta, immune cells, liver, bone growth plates and several other endocrine tissues. Subunit expression varies between 'normal' tissue and malignancies, as GABAA receptors can influence cell proliferation.[45]
| Isoform | Synaptic/Extrasynaptic | Anatomical location |
|---|---|---|
| α1β3γ2S | Both | Widespread |
| α2β3γ2S | Both | Widespread |
| α3β3γ2S | Both | Reticular thalamic nucleus |
| α4β3γ2S | Both | Thalamic relay cells |
| α5β3γ2S | Both | Hippocampal pyramidal cells |
| α6β3γ2S | Both | Cerebellar granule cells |
| α1β2γ2S | Both | Widespread, most abundant |
| α4β3δ | Extrasynaptic | Thalamic relay cells |
| α6β3δ | Extrasynaptic | Cerebellar granule cells |
| α1β2 | Extrasynaptic | Widespread |
| α1β3 | Extrasynaptic | Thalamus, hypothalamus |
| α1β2δ | Extrasynaptic | Hippocampus |
| α4β2δ | Extrasynaptic | Hippocampus, Prefrontal cortex |
| α3β3θ | Extrasynaptic | Hypothalamus |
| α3β3ε | Extrasynaptic | Hypothalamus |
Ligands

A number of ligands have been found to bind to various sites on the GABAA receptor complex and modulate it besides GABA itself.[which?] A ligand can possess one or more properties of the following types. Unfortunately the literature often does not distinguish these types properly.
Types
- Orthosteric agonists and antagonists: bind to the main receptor site (the site where GABA normally binds, also referred to as the "active" or "orthosteric" site). Agonists activate the receptor, resulting in increased Cl− conductance. Antagonists, though they have no effect on their own, compete with GABA for binding and thereby inhibit its action, resulting in decreased Cl− conductance.
- First order allosteric modulators: bind to allosteric sites on the receptor complex and affect it either in a positive (PAM), negative (NAM) or neutral/silent (SAM) manner, causing increased or decreased efficiency of the main site and therefore an indirect increase or decrease in Cl− conductance. SAMs do not affect the conductance, but occupy the binding site.
- Second order modulators: bind to an allosteric site on the receptor complex and modulate the effect of first order modulators.
- Open channel blockers: prolong ligand-receptor occupancy, activation kinetics and Cl ion flux in a subunit configuration-dependent and sensitization-state dependent manner.[47]
- Non-competitive channel blockers: bind to or near the central pore of the receptor complex and directly block Cl− conductance through the ion channel.
Examples
- Orthosteric agonists: GABA, gaboxadol, isoguvacine, muscimol, progabide, beta-alanine,[48][49] taurine,[49][48] piperidine-4-sulfonic acid (partial agonist).
- Orthosteric antagonists: bicuculline, gabazine.
- Positive allosteric modulators: barbiturates, benzodiazepines, certain carbamates (ex. carisoprodol, meprobamate, lorbamate), Honokiol, Magnolol, Baicalin, Baicelin, thienodiazepines, alcohol (ethanol), etomidate, glutethimide, kavalactones,[50] meprobamate, quinazolinones (ex. methaqualone, etaqualone, diproqualone), neuroactive steroids,[51] niacin/niacinamide,[52] nonbenzodiazepines (ex. zolpidem, eszopiclone), propofol, stiripentol,[53] theanine,[citation needed] valerenic acid, volatile/inhaled anesthetics, lanthanum,[54] riluzole,[55] and menthol.[56]
- Negative allosteric modulators: flumazenil, Ro15-4513, sarmazenil, Pregnenolone sulfate, amentoflavone, and zinc.[57]
- Inverse allosteric agonists: beta-carbolines (ex. Harmine, Harmaline, Tetrahydroharmine).
- Second-order modulators: (−)‐epigallocatechin‐3‐gallate.[58]
- Non-competitive channel blockers: cicutoxin, oenanthotoxin, pentylenetetrazol, picrotoxin[citation needed], thujone, and lindane.
Effects
Ligands which contribute to receptor activation typically have anxiolytic, anticonvulsant, amnesic, sedative, hypnotic, euphoriant, and muscle relaxant properties. Some such as muscimol and the z-drugs may also be hallucinogenic.[citation needed] Ligands which decrease receptor activation usually have opposite effects, including anxiogenesis and convulsion.[citation needed] Some of the subtype-selective negative allosteric modulators such as α5IA are being investigated for their nootropic effects, as well as treatments for the unwanted side effects of other GABAergic drugs.[59] Advances in molecular pharmacology and genetic manipulation of rat genes have revealed that distinct subtypes of the GABAA receptor mediate certain parts of the anaesthetic behavioral repertoire.[60]
Novel drugs
A useful property of the many benzodiazepine site allosteric modulators is that they may display selective binding to particular subsets of receptors comprising specific subunits. This allows one to determine which GABAA receptor subunit combinations are prevalent in particular brain areas and provides a clue as to which subunit combinations may be responsible for behavioral effects of drugs acting at GABAA receptors. These selective ligands may have pharmacological advantages in that they may allow dissociation of desired therapeutic effects from undesirable side effects.[61] Few subtype selective ligands have gone into clinical use as yet, with the exception of zolpidem which is reasonably selective for α1, but several more selective compounds are in development such as the α3-selective drug adipiplon. There are many examples of subtype-selective compounds which are widely used in scientific research, including:
Diazepam is a benzodiazepine medication that is FDA approved for the treatment of anxiety disorders, the short-term relief of anxiety symptoms, spasticity associated with upper motor neuron disorders, adjunct therapy for muscle spasms, preoperative anxiety relief, the management of certain refractory epileptic patients, and as an adjunct in severe recurrent convulsive seizures and status epilepticus.[62]
- CL-218,872 (highly α1-selective agonist)
- bretazenil (subtype-selective partial agonist)
- imidazenil and L-838,417 (both partial agonists at some subtypes, but weak antagonists at others)
- QH-ii-066 (full agonist highly selective for α5 subtype)
- α5IA (selective inverse agonist for α5 subtype)
- SL-651,498 (full agonist at α2 and α3 subtypes, and as a partial agonist at α1 and α5
- 3-acyl-4-quinolones: selective for α1 over α3[63]
Paradoxical reactions
There are multiple indications that paradoxical reactions upon – for example – benzodiazepines, barbiturates, inhalational anesthetics, propofol, neurosteroids, and alcohol are associated with structural deviations of GABAA receptors. The combination of the five subunits of the receptor (see images above) can be altered in such a way that for example the receptor's response to GABA remains unchanged but the response to one of the named substances is dramatically different from the normal one.
There are estimates that about 2–3 % of the general population may suffer from serious emotional disorders due to such receptor deviations, with up to 20% suffering from moderate disorders of this kind. It is generally assumed that the receptor alterations are, at least partly, due to genetic and also epigenetic deviations. There are indication that the latter may be triggered by, among other factors, social stress or occupational burnout.[64][65][66][67]
See also
- 4-Iodopropofol
- GABA receptor
- GABAB receptor
- GABAA-ρ receptor
- Gephyrin
- Glycine receptor
- GABAA receptor positive allosteric modulators
- GABAA receptor negative allosteric modulators
References
- ↑ Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011 May 12;70(3):385-409. doi: 10.1016/j.neuron.2011.03.024. PMID: 21555068; PMCID: PMC3093971.
- ↑ The Oxford handbook of stress, health, and coping. Folkman, Susan.. Oxford: Oxford University Press. 2011. ISBN 978-0-19-537534-3. OCLC 540015689.
- ↑ "Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance". Nature 330 (6144): 163–5. 18 November 1987. doi:10.1038/330163a0. PMID 3670401. Bibcode: 1987Natur.330..163K.
- ↑ Olsen RW. GABAA receptor: Positive and negative allosteric modulators. Neuropharmacology. 2018 Jul 1;136(Pt A):10-22. doi: 10.1016/j.neuropharm.2018.01.036. Epub 2018 Jan 31. PMID: 29407219; PMCID: PMC6027637.
- ↑ Olsen RW. GABAA receptor: Positive and negative allosteric modulators. Neuropharmacology. 2018 Jul 1;136(Pt A):10-22. doi: 10.1016/j.neuropharm.2018.01.036. Epub 2018 Jan 31. PMID: 29407219; PMCID: PMC6027637.
- ↑ Principles of neural science. Kandel, Eric R.,, Schwartz, James H. (James Harris), 1932-2006,, Jessell, Thomas M.,, Siegelbaum, Steven,, Hudspeth, A. James,, Mack, Sarah (5th ed.). New York. ISBN 978-1-283-65624-5. OCLC 919404585.
- ↑ "GABA a Receptors and the Diversity in their Structure and Pharmacology". GABAA Receptors and the Diversity in their Structure and Pharmacology. Advances in Pharmacology. 79. 2017. pp. 1–34. doi:10.1016/bs.apha.2017.03.003. ISBN 978-0-12-810413-2.
- ↑ "Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition". Alcohol 41 (3): 211–221. May 2007. doi:10.1016/j.alcohol.2007.04.011. PMID 17591544.
- ↑ "GABAA receptor pharmacology". Pharmacology & Therapeutics 69 (3): 173–198. 1996. doi:10.1016/0163-7258(95)02043-8. PMID 8783370.
- ↑ Evenseth LSM, Gabrielsen M, Sylte I. The GABAB Receptor-Structure, Ligand Binding and Drug Development. Molecules. 2020 Jul 7;25(13):3093. doi: 10.3390/molecules25133093. PMID: 32646032; PMCID: PMC7411975.
- ↑ "Mapping of the benzodiazepine recognition site on GABA(A) receptors". Current Topics in Medicinal Chemistry 2 (8): 833–839. August 2002. doi:10.2174/1568026023393444. PMID 12171574.
- ↑ GABAA receptor structure-function studies: a reexamination in light of new acetylcholine receptor structures. International Review of Neurobiology. 62. 2004. pp. 1–43. doi:10.1016/S0074-7742(04)62001-0. ISBN 978-0-12-366862-2.
- ↑ "Identification of a residue in the gamma-aminobutyric acid type A receptor alpha subunit that differentially affects diazepam-sensitive and -insensitive benzodiazepine site binding". Journal of Neurochemistry 88 (6): 1431–1438. March 2004. doi:10.1046/j.1471-4159.2003.02264.x. PMID 15009644.
- ↑ "International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function". Pharmacological Reviews 50 (2): 291–313. June 1998. PMID 9647870. http://pharmrev.aspetjournals.org/cgi/content/abstract/50/2/291.
- ↑ Gidal B, Detyniecki K. Rescue therapies for seizure clusters: Pharmacology and target of treatments. Epilepsia. 2022 Sep;63 Suppl 1 (Suppl 1):S34-S44. doi: 10.1111/epi.17341. PMID: 35999174; PMCID: PMC9543841.
- ↑ "Cryo-EM structure of the benzodiazepine-sensitive α1β1γ2S tri-heteromeric GABAA receptor in complex with GABA". eLife 7: e39383. July 2018. doi:10.7554/eLife.39383. PMID 30044221.
- ↑ "Anxioselective compounds acting at the GABA(A) receptor benzodiazepine binding site". Current Drug Targets. CNS and Neurological Disorders 2 (4): 213–232. August 2003. doi:10.2174/1568007033482841. PMID 12871032.
- ↑ "Structural mechanisms underlying benzodiazepine modulation of the GABA(A) receptor". The Journal of Neuroscience 28 (13): 3490–3499. March 2008. doi:10.1523/JNEUROSCI.5727-07.2008. PMID 18367615.
- ↑ "Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital". Annals of Neurology 25 (3): 213–220. March 1989. doi:10.1002/ana.410250302. PMID 2471436.
- ↑ Hanson SM, Czajkowski C. Structural mechanisms underlying benzodiazepine modulation of the GABA(A) receptor. J Neurosci. 2008 Mar 26;28(13):3490-9. doi: 10.1523/JNEUROSCI.5727-07.2008. PMID: 18367615; PMCID: PMC2410040.
- ↑ "Diazepam-bound GABAA receptor models identify new benzodiazepine binding-site ligands". Nature Chemical Biology 8 (5): 455–464. March 2012. doi:10.1038/nchembio.917. PMID 22446838.
- ↑ "Comparative models of GABAA receptor extracellular and transmembrane domains: important insights in pharmacology and function". Molecular Pharmacology 68 (5): 1291–1300. November 2005. doi:10.1124/mol.105.015982. PMID 16103045. http://pdfs.semanticscholar.org/c200/428f6c9e06f04a085de7868e10242f1823ac.pdf.
- ↑ "Modeling the closed and open state conformations of the GABA(A) ion channel--plausible structural insights for channel gating". Journal of Chemical Information and Modeling 52 (11): 2958–2969. November 2012. doi:10.1021/ci300189a. PMID 23116339.
- ↑ "Exploring ligand recognition and ion flow in comparative models of the human GABA type A receptor". Journal of Molecular Graphics and Modelling 26 (4): 760–774. November 2007. doi:10.1016/j.jmgm.2007.04.012. PMID 17544304.
- ↑ "Crystal structure of a human GABAA receptor". Nature 512 (7514): 270–275. August 2014. doi:10.1038/nature13293. PMID 24909990. Bibcode: 2014Natur.512..270M.
- ↑ "Cryo-EM structure of the benzodiazepine-sensitive α1β1γ2S tri-heteromeric GABAA receptor in complex with GABA". eLife 7: e39383. July 2018. doi:10.7554/eLife.39383. PMID 30044221.
- ↑ "Structure of a human synaptic GABAA receptor". Nature 559 (7712): 67–72. July 2018. doi:10.1038/s41586-018-0255-3. PMID 29950725. Bibcode: 2018Natur.559...67Z.
- ↑ "Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus". The Journal of Neuroscience 23 (33): 10650–61. November 2003. doi:10.1523/JNEUROSCI.23-33-10650.2003. PMID 14627650.
- ↑ "Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors". Nature Reviews. Neuroscience 6 (3): 215–29. March 2005. doi:10.1038/nrn1625. PMID 15738957.
- ↑ "Functional movements of the GABA type A receptor". Physical Chemistry Chemical Physics 22 (28): 16023–16031. July 2020. doi:10.1039/D0CP01128B. PMID 32633279. Bibcode: 2020PCCP...2216023V.
- ↑ "Giant synaptic potentials in immature rat CA3 hippocampal neurones". The Journal of Physiology 416: 303–325. September 1989. doi:10.1113/jphysiol.1989.sp017762. PMID 2575165.
- ↑ "How GABA generates depolarization". The Journal of Physiology 588 (Pt 5): 757–758. March 2010. doi:10.1113/jphysiol.2009.183574. PMID 20194137.
- ↑ "Chapter 16: GABA and Glycine". Basic neurochemistry: molecular, cellular, and medical aspects (Sixth ed.). Philadelphia: Lippincott-Raven. 1999. ISBN 978-0-397-51820-3. https://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=bnchm.section.1181.
- ↑ "Role of GABAB receptors in GABA and baclofen-induced inhibition of adult rat cerebellar interpositus nucleus neurons in vitro". Brain Research Bulletin 67 (4): 310–318. October 2005. doi:10.1016/j.brainresbull.2005.07.004. PMID 16182939.
- ↑ "Identification of inosine as an endogenous modulator for the benzodiazepine binding site of the GABAA receptors". Journal of Biomedical Science 5 (4): 274–280. 2016-08-01. doi:10.1007/bf02255859. PMID 9691220.
- ↑ Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011 May 12;70(3):385-409. doi: 10.1016/j.neuron.2011.03.024. PMID: 21555068; PMCID: PMC3093971.
- ↑ Braat S, Kooy RF. The GABAA Receptor as a Therapeutic Target for Neurodevelopmental Disorders. Neuron. 2015 Jun 3;86(5):1119-30. doi: 10.1016/j.neuron.2015.03.042. PMID: 26050032.
- ↑ "Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies". Trends in Neurosciences 28 (2): 108–115. February 2005. doi:10.1016/j.tins.2004.11.011. PMID 15667934.
- ↑ "Molecular composition of GABAC receptors". Vision Research 38 (10): 1431–1441. May 1998. doi:10.1016/S0042-6989(97)00277-0. PMID 9667009.
- ↑ "GABA A receptors: subtypes provide diversity of function and pharmacology". Neuropharmacology 56 (1): 141–148. January 2009. doi:10.1016/j.neuropharm.2008.07.045. PMID 18760291.
- ↑ "Cell engineering method using fluorogenic oligonucleotide signaling probes and flow cytometry". Biotechnology Letters 43 (5): 949–958. March 2021. doi:10.1007/s10529-021-03101-5. PMID 33683511.
- ↑ "Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors". The Journal of Biological Chemistry 271 (1): 89–96. January 1996. doi:10.1074/jbc.271.1.89. PMID 8550630.
- ↑ "Differential organization of gamma-aminobutyric acid type A and glycine receptors in the somatic and dendritic compartments of rat abducens motoneurons". The Journal of Comparative Neurology 504 (2): 112–126. September 2007. doi:10.1002/cne.21442. PMID 17626281.
- ↑ Macdonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1995;36 Suppl 2:S2-12. doi: 10.1111/j.1528-1157.1995. tb05996.x. PMID: 8784210.
- ↑ ten Hoeve AL (2012). GABA receptors and the immune system . Thesis, Utrecht University
- ↑ "GABA Potency at GABA(A) Receptors Found in Synaptic and Extrasynaptic Zones" (in en). Frontiers in Cellular Neuroscience 6: 1. January 2011. doi:10.3389/fncel.2012.00001. PMID 22319471.
- ↑ "GABA(A) receptor activation and open-channel block by volatile anaesthetics: a new principle of receptor modulation?". European Journal of Pharmacology 451 (1): 43–50. September 2002. doi:10.1016/S0014-2999(02)02194-5. PMID 12223227.
- ↑ 48.0 48.1 "Beta-alanine and taurine as endogenous agonists at glycine receptors in rat hippocampus in vitro". The Journal of Physiology 539 (Pt 1): 191–200. February 2002. doi:10.1113/jphysiol.2001.013147. PMID 11850512.
- ↑ 49.0 49.1 "Taurine and beta-alanine act on both GABA and glycine receptors in Xenopus oocyte injected with mouse brain messenger RNA". Brain Research 464 (2): 97–105. September 1988. doi:10.1016/0169-328x(88)90002-2. PMID 2464409.
- ↑ Hunter, A (2006). "Kava (Piper methysticum) back in circulation". Australian Centre for Complementary Medicine 25 (7): 529.
- ↑ (a) "Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors". Pharmacology & Therapeutics 116 (1): 20–34. October 2007. doi:10.1016/j.pharmthera.2007.03.007. PMID 17531325. http://www.journals.elsevier.com/pharmacology-and-therapeutics.; (b) "Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites". Nature 444 (7118): 486–489. November 2006. doi:10.1038/nature05324. PMID 17108970. Bibcode: 2006Natur.444..486H.; (c)"Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis". Proceedings of the National Academy of Sciences of the United States of America 103 (39): 14602–14607. September 2006. doi:10.1073/pnas.0606544103. PMID 16984997. Bibcode: 2006PNAS..10314602A.; (d) "Neurosteroid access to the GABAA receptor". The Journal of Neuroscience 25 (50): 11605–11613. December 2005. doi:10.1523/JNEUROSCI.4173-05.2005. PMID 16354918.; (e) "Neurosteroids: endogenous regulators of the GABA(A) receptor". Nature Reviews. Neuroscience 6 (7): 565–575. July 2005. doi:10.1038/nrn1703. PMID 15959466.; (f) "Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake". Psychopharmacology 186 (3): 362–372. June 2006. doi:10.1007/s00213-005-0213-2. PMID 16432684.; (g) "Steroids, neuroactive steroids and neurosteroids in psychopathology". Progress in Neuro-Psychopharmacology & Biological Psychiatry 29 (2): 169–192. February 2005. doi:10.1016/j.pnpbp.2004.11.001. PMID 15694225.; (h) "Neurosteroids: biochemistry and clinical significance". Trends in Endocrinology and Metabolism 13 (1): 35–43. 2002. doi:10.1016/S1043-2760(01)00503-3. PMID 11750861.; (i) "Neurosteroids act on recombinant human GABAA receptors". Neuron 4 (5): 759–765. May 1990. doi:10.1016/0896-6273(90)90202-Q. PMID 2160838.; (j) "Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor". Science 232 (4753): 1004–1007. May 1986. doi:10.1126/science.2422758. PMID 2422758. Bibcode: 1986Sci...232.1004D. https://zenodo.org/record/1230988.; (k) "Neurosteroids — Endogenous Regulators of Seizure Susceptibility and Role in the Treatment of Epilepsy". Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US). National Center for Biotechnology Information (US). 2012. https://www.ncbi.nlm.nih.gov/books/NBK98218/.
- ↑ "STUDY OF GABAERGIC AGONISTS". Deccan Journal of Pharmacology 1 (2): 56–69. 2010. http://www.ijdpls.com/uploaded/journal_files/120402040442.pdf. Retrieved 2013-02-12.
- ↑ "The anti-convulsant stiripentol acts directly on the GABA(A) receptor as a positive allosteric modulator". Neuropharmacology 56 (1): 190–197. January 2009. doi:10.1016/j.neuropharm.2008.06.004. PMID 18585399.
- ↑ "Lanthanum potentiates GABA-activated currents in rat pyramidal neurons of CA1 hippocampal field". Bulletin of Experimental Biology and Medicine 140 (4): 403–405. October 2005. doi:10.1007/s10517-005-0503-z. PMID 16671565.
- ↑ "Neuroprotective agent riluzole potentiates postsynaptic GABA(A) receptor function". Neuropharmacology 42 (2): 199–209. February 2002. doi:10.1016/s0028-3908(01)00175-7. PMID 11804616.
- ↑ Lau, B. K.; Karim, S.; Goodchild, A. K.; Vaughan, C. W.; Drew, G. M. (2014). "Menthol enhances phasic and tonic GABAA receptor-mediated currents in midbrain periaqueductal grey neurons". British Journal of Pharmacology 171 (11): 2803–2813. doi:10.1111/bph.12602. PMID 24460753.
- ↑ "Zinc-mediated inhibition of GABA(A) receptors: discrete binding sites underlie subtype specificity". Nature Neuroscience 6 (4): 362–369. April 2003. doi:10.1038/nn1030. PMID 12640458.
- ↑ "The dietary flavonoids apigenin and (-)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABA(A) receptors". Biochemical Pharmacology. Six Decades of GABA 68 (8): 1631–8. October 2004. doi:10.1016/j.bcp.2004.07.022. PMID 15451406.
- ↑ "An inverse agonist selective for alpha5 subunit-containing GABAA receptors enhances cognition". The Journal of Pharmacology and Experimental Therapeutics 316 (3): 1335–1345. March 2006. doi:10.1124/jpet.105.092320. PMID 16326923. http://pdfs.semanticscholar.org/22aa/5af270a5dff6b125aadd1c231dd0bd464782.pdf.
- ↑ Weir CJ, Mitchell SJ, Lambert JJ. Role of GABAA receptor subtypes in the behavioural effects of intravenous general anaesthetics. Br J Anaesth. 2017 Dec 1;119(suppl_1):i167-i175. doi: 10.1093/bja/aex369. PMID: 29161398.
- ↑ "GABA A/Bz receptor subtypes as targets for selective drugs". Current Medicinal Chemistry 14 (25): 2680–2701. 2007. doi:10.2174/092986707782023190. PMID 17979718.
- ↑ Sieghart W, Ramerstorfer J, Sarto-Jackson I, Varagic Z, Ernst M. A novel GABA(A) receptor pharmacology: drugs interacting with the α(+) β(-) interface. Br J Pharmacol. 2012 May;166(2):476-85. doi: 10.1111/j.1476-5381.2011.01779.x. PMID: 22074382; PMCID: PMC3417481.
- ↑ "Affinity of 3-acyl substituted 4-quinolones at the benzodiazepine site of GABA(A) receptors". Bioorganic & Medicinal Chemistry 16 (14): 6936–6948. July 2008. doi:10.1016/j.bmc.2008.05.049. PMID 18541432.
- ↑ "Paradoxical reactions to benzodiazepines in intravenous sedation: a report of 2 cases and review of the literature". Anesthesia Progress 49 (4): 128–32. 2002. PMID 12779114.
- ↑ "Benzodiazepines and disinhibition: a review". Psychiatric Bulletin (Royal College of Psychiatrists) 26 (12): 460–462. 2002. doi:10.1192/pb.26.12.460. ISSN 0955-6036. https://www.cambridge.org/core/services/aop-cambridge-core/content/view/421AF197362B55EDF004700452BF3BC6/S0955603600001240a.pdf/benzodiazepines_and_disinhibition_a_review.pdf.
- ↑ "Allopregnanolone and mood disorders". Progress in Neurobiology 113: 88–94. February 2014. doi:10.1016/j.pneurobio.2013.07.005. PMID 23978486.
- ↑ "General anesthesia, sleep, and coma". The New England Journal of Medicine 363 (27): 2638–50. December 2010. doi:10.1056/NEJMra0808281. PMID 21190458.
Further reading
- "Chapter 16: GABA and Glycine". Basic neurochemistry: molecular, cellular, and medical aspects (Sixth ed.). Philadelphia: Lippincott-Raven. 1999. ISBN 978-0-397-51820-3. https://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=bnchm.section.1181.
- "Chapter 16: GABA and Glycine". Basic Neurochemistry: Molecular, Cellular and Medical Aspects (Seventh ed.). Boston: Academic Press. 2005. pp. 291–302. ISBN 978-0-12-088397-4.
- "Regulation of GABA(A) receptor subunit expression by pharmacological agents". Pharmacological Reviews 62 (1): 97–135. March 2010. doi:10.1124/pr.109.002063. PMID 20123953. http://pdfs.semanticscholar.org/454d/e49fb200bcbbe32c3c5c15da71381eef2e54.pdf.
- Rudolph U (2015). Diversity and Functions of GABA Receptors: A Tribute to Hanns Möhler (First ed.). Academic Press, Elsevier. ISBN 978-0-12-802660-1.
External links
- Receptors,+GABA-A at the US National Library of Medicine Medical Subject Headings (MeSH)
{{Navbox | name = GABA receptor modulators | title = GABA receptor modulators | state = collapsed | bodyclass = hlist | groupstyle = text-align:center;
| group1 = Ionotropic | list1 = {{Navbox|subgroup | groupstyle = text-align:center | groupwidth = 5em
| group1 = GABAA | list1 =
- Agonists: (+)-Catechin
- Bamaluzole
- Barbiturates (e.g., phenobarbital)
- BL-1020
- DAVA
- Dihydromuscimol
- GABA
- Gabamide
- GABOB
- Gaboxadol (THIP)
- Homotaurine (tramiprosate, 3-APS)
- Ibotenic acid
- iso-THAZ
- iso-THIP
- Isoguvacine
- Isomuscimol
- Isonipecotic acid
- Kojic amine
- Lignans (e.g., honokiol)
- Methylglyoxal
- Monastrol
- Muscimol
- Nefiracetam
- Neuroactive steroids (e.g., allopregnanolone)
- Org 20599
- PF-6372865
- Phenibut
- Picamilon
- P4S
- Progabide
- Propofol
- Quisqualamine
- SL-75102
- TACA
- TAMP
- Terpenoids (e.g., borneol)
- Thiomuscimol
- Tolgabide
- ZAPA
- Positive modulators (abridged; see here for a full list): α-EMTBL
- Alcohols (e.g., ethanol)
- Anabolic steroids
- Avermectins (e.g., ivermectin)
- Barbiturates (e.g., phenobarbital)
- Benzodiazepines (e.g., diazepam)
- Bromide compounds (e.g., potassium bromide)
- Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
- Fenamates (e.g., mefenamic acid)
- Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
- Imidazoles (e.g., etomidate)
- Kava constituents (e.g., kavain)<!--PMID: 9776662-->
- Lanthanum
- Loreclezole
- Monastrol
- Neuroactive steroids (e.g., allopregnanolone, [[Chemistry:Cholecholesterol]], THDOC)
- Niacin
- Nicotinamide (niacinamide)
- Nonbenzodiazepines (e.g., β-carbolines (e.g., [[abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
- Phenols (e.g., propofol)
- Phenytoin
- Piperidinediones (e.g., glutethimide)
- Propanidid
- Pyrazolopyridines (e.g., etazolate)
- Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
- Skullcap constituents (e.g., baicalin)
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
- Valerian constituents (e.g., valerenic acid)
- Volatiles/gases (e.g., chloral hydrate, chloroform, [[Chemistry:Diethyl diethyl ether, Parparaldehyde]], sevoflurane)
- Antagonists: Bicuculline
- Coriamyrtin
- Dihydrosecurinine
- Gabazine (SR-95531)
- Hydrastine
- Hyenachin (mellitoxin)
- PHP-501
- Pitrazepin
- Securinine
- Sinomenine
- SR-42641
- SR-95103
- Thiocolchicoside
- Tutin
- Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
- β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
- Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
- Fiproles (e.g., fipronil)
- Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
- Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Golexanolone
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
- Nonsteroidal antiandrogens (e.g., [[apalutamide, [[Chemistry:Bicalutbicalutamide, Enzalutenzalutamide, Chemistry:Flutamide|flut]]amide]], nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
- Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
| group2 = GABAA-ρ | list2 =
- Agonists: BL-1020
- CACA
- CAMP
- Homohypotaurine
- GABA
- GABOB
- Ibotenic acid
- Isoguvacine
- Muscimol
- N4-Chloroacetylcytosine arabinoside
- Picamilon
- Progabide
- TACA
- TAMP
- Thiomuscimol
- Tolgabide
- Positive modulators: Allopregnanolone
- Alphaxolone
- ATHDOC
- Lanthanides
- Antagonists: (S)-2-MeGABA
- (S)-4-ACPBPA
- (S)-4-ACPCA
- 2-MeTACA
- 3-APMPA
- 4-ACPAM
- 4-GBA
- cis-3-ACPBPA
- CGP-36742 (SGS-742)
- DAVA
- Gabazine (SR-95531)
- Gaboxadol (THIP)
- I4AA
- Isonipecotic acid
- Loreclezole
- P4MPA
- P4S
- SKF-97541
- SR-95318
- SR-95813
- TPMPA
- trans-3-ACPBPA
- ZAPA
- Negative modulators: 5α-Dihydroprogesterone
- Bilobalide
- Loreclezole
- Picrotoxin (picrotin, picrotoxinin)
- Pregnanolone
- ROD-188
- THDOC
- Zinc
}}
| group2 = Metabotropic
| list2 =
| below =
- See also
- Receptor/signaling modulators
- GABAA receptor positive modulators
- GABA metabolism/transport modulators
}}
 |
