Biology:XPO5
Exportin-5 (XPO5) is a protein that, in humans, is encoded by the XPO5 gene.[1][2][3] In eukaryotic cells, the primary purpose of XPO5 is to export pre-microRNA (also known as pre-miRNA) out of the nucleus and into the cytoplasm, for further processing by the Dicer enzyme.[4][5][6][7] Once in the cytoplasm, the microRNA (also known as miRNA) can act as a gene silencer by regulating translation of mRNA. Although XPO5 is primarily involved in the transport of pre-miRNA, it has also been reported to transport tRNA.[8]
Much research on XPO5 is ongoing. miRNA is a prominent research topic due to its potential use as a therapeutic, with several miRNA-based drugs already in use.[9]
Mechanism
Binding to pre-miRNA
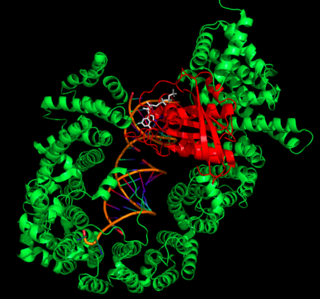
After RanGTP binds to XPO5, the XPO5-RanGTP complex forms a U-like structure to hold the pre-miRNA. The XPO5-RanGTP complex recognizes pre-miRNA by its two-nucleotide 3’ overhang—a sequence consisting of two bases at the 3’ end of the pre-miRNA that are not paired with other bases. This motif is unique to pre-miRNA, and by recognizing it XPO5 ensures specificity for transporting only pre-miRNA. On its own, pre-miRNA is in a “closed” conformation, with the 3’ overhang flipped up toward the RNA minor groove. However, upon binding to XPO5, the 3’ overhang is flipped downwards away from the rest of the pre-miRNA molecule into an “open” conformation. This helps the backbone phosphates of these two nucleotides form hydrogen bonds with many XPO5 residues, allowing XPO5 to recognize the RNA as pre-miRNA. Because these interactions involve only the RNA phosphate backbone, they are nonspecific and allow XPO5 to recognize and transport any pre-miRNA. The rest of the pre-miRNA stem binds to XPO5 via interactions between the negatively-charged phosphate backbone and several positively-charged interior XPO5 residues.[11]
XPO5 Ternary Complex Transport Mechanism
The combined structure of XPO5, RanGTP, and pre-miRNA is known as the ternary complex. Once the ternary complex is formed, it diffuses through a nuclear pore complex into the cytoplasm, transporting pre-miRNA into the cytoplasm in the process. Once in the cytoplasm, RanGAP hydrolyzes GTP to GDP, causing a conformational change that releases the pre-miRNA into the cytoplasm.[11]
Export out of the Nucleus
It has been suggested, through evidence provided by contour maps of water density, that the interior of XPO5 is hydrophilic, while the exterior of XPO5 is hydrophobic.[11] Therefore, this enhances the binding capabilities of XPO5 to the nuclear pore complex, allowing for transport of the ternary complex out of the nucleus.[11]
Additional interactions
XPO5 has been shown to interact with ILF3[1] and Ran.[1]
Potential oncogenic role
Recent evidence has shown higher levels of XPO5 in prostate cancer cell lines in-vitro, suggesting that altered XPO5 expression levels may have a role in cancer development. Suppressing XPO5 has also been found to be therapeutic in-vitro.[12] It has also been shown to function as an oncogene in colorectal cancer.[13]
References
- ↑ Jump up to: 1.0 1.1 1.2 "Exportin-5, a novel karyopherin, mediates nuclear export of double-stranded RNA binding proteins". The Journal of Cell Biology 156 (1): 53–64. Jan 2002. doi:10.1083/jcb.200110082. PMID 11777942.
- ↑ "Exp5 exports eEF1A via tRNA from nuclei and synergizes with other transport pathways to confine translation to the cytoplasm". The EMBO Journal 21 (22): 6205–15. Nov 2002. doi:10.1093/emboj/cdf613. PMID 12426392.
- ↑ "Entrez Gene: XPO5 exportin 5". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=57510.
- ↑ "Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs". Genes & Development 17 (24): 3011–6. Dec 2003. doi:10.1101/gad.1158803. PMID 14681208.
- ↑ "Molecular mechanisms of RNA interference". Annual Review of Biophysics 42: 217–39. 2013. doi:10.1146/annurev-biophys-083012-130404. PMID 23654304.
- ↑ "Posttranscriptional regulation of microRNA biogenesis in animals". Molecular Cell 38 (3): 323–32. May 2010. doi:10.1016/j.molcel.2010.03.013. PMID 20471939.
- ↑ "Cellular functions of the microprocessor". Biochemical Society Transactions 41 (4): 838–43. Aug 2013. doi:10.1042/BST20130011. PMID 23863141.
- ↑ Gupta, Asmita (2016). "Insights into the Structural Dynamics of Nucleocytoplasmic Transport of tRNA by Exportin-t". Biophysical Journal 110 (6): 1264–1279. doi:10.1016/j.bpj.2016.02.015. PMID 27028637. Bibcode: 2016BpJ...110.1264G.
- ↑ Christopher, Ajay Francis; Kaur, Raman Preet; Kaur, Gunpreet; Kaur, Amandeep; Gupta, Vikas; Bansal, Parveen (2016). "MicroRNA therapeutics: Discovering novel targets and developing specific therapy". Perspectives in Clinical Research 7 (2): 68–74. doi:10.4103/2229-3485.179431. ISSN 2229-3485. PMID 27141472.
- ↑ Okada, Chimari; Yamashita, Eiki; Lee, Soo Jae; Shibata, Satoshi; Katahira, Jun; Nakagawa, Atsushi; Yoneda, Yoshihiro; Tsukihara, Tomitake (2009-11-27). "A high-resolution structure of the pre-microRNA nuclear export machinery". Science 326 (5957): 1275–1279. doi:10.1126/science.1178705. ISSN 1095-9203. PMID 19965479. Bibcode: 2009Sci...326.1275O.
- ↑ Jump up to: 11.0 11.1 11.2 11.3 Wang, Xia (2011). "Dynamic mechanisms for pre-miRNA binding and export by Exportin-5". RNA 17 (8): 1516–1517. doi:10.1261/rna.2732611. PMID 21712399.
- ↑ Höti, Naseruddin; Yang, Shuang; Aiyetan, Paul; Kumar, Binod; Hu, Yingwei; Clark, David; Eroglu, Arife Unal; Shah, Punit et al. (2017-09-04). "Overexpression of Exportin-5 Overrides the Inhibitory Effect of miRNAs Regulation Control and Stabilize Proteins via Posttranslation Modifications in Prostate Cancer". Neoplasia (New York, N.Y.) 19 (10): 817–829. doi:10.1016/j.neo.2017.07.008. ISSN 1522-8002. PMID 28881308.
- ↑ Shigeyasu, Kunitoshi; Okugawa, Yoshinaga; Toden, Shusuke; Boland, C. Richard; Goel, Ajay (2017-03-01). "Exportin-5 Functions as an Oncogene and a Potential Therapeutic Target in Colorectal Cancer". Clinical Cancer Research 23 (5): 1312–1322. doi:10.1158/1078-0432.CCR-16-1023. ISSN 1557-3265. PMID 27553833.
Further reading
- "Exportin-5-mediated nuclear export of eukaryotic elongation factor 1A and tRNA". The EMBO Journal 21 (22): 6216–24. Nov 2002. doi:10.1093/emboj/cdf620. PMID 12426393.
- "Exportin-5 mediates nuclear export of minihelix-containing RNAs". The Journal of Biological Chemistry 278 (8): 5505–8. Feb 2003. doi:10.1074/jbc.C200668200. PMID 12509441.
- "Minihelix-containing RNAs mediate exportin-5-dependent nuclear export of the double-stranded RNA-binding protein ILF3". The Journal of Biological Chemistry 279 (2): 884–91. Jan 2004. doi:10.1074/jbc.M306808200. PMID 14570900.
- "Nuclear export of microRNA precursors". Science 303 (5654): 95–8. Jan 2004. doi:10.1126/science.1090599. PMID 14631048. Bibcode: 2004Sci...303...95L.
- "Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs". RNA 10 (2): 185–91. Feb 2004. doi:10.1261/rna.5167604. PMID 14730017.
- "Nucleocytoplasmic shuttling of JAZ, a new cargo protein for exportin-5". Molecular and Cellular Biology 24 (15): 6608–19. Aug 2004. doi:10.1128/MCB.24.15.6608-6619.2004. PMID 15254228.
- "Overexpression of exportin 5 enhances RNA interference mediated by short hairpin RNAs and microRNAs". RNA 11 (2): 220–6. Feb 2005. doi:10.1261/rna.7233305. PMID 15613540.
 |


