Chemistry:(2.2)Paracyclophane
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Tricyclo[8.2.2.24,7]hexadeca-4,6,10,12,13,15-hexaene
| |
| Other names
[2.2](1,4)Cyclophane
1,4-Carbophane Cyclobis(benzene-1,4-dimethylene) Parylene dimer Di-p-xylylene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| Properties | |
| C16H16 | |
| Molar mass | 208.304 g·mol−1 |
| Appearance | White solid[1] |
| Density | 1.242 g/cm3 (260 K)[2] |
| Melting point | 285 °C (545 °F; 558 K)[3] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H317, H373 | |
| P260, P261, P272, P280, P302+352, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P333+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
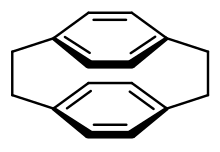
[2.2]Paracyclophane is a cyclophane that is applied in bio- and materials science. It was first synthesized by Brown and Farthing in 1949 by pyrolyzing para-xylene in the gas phase under low pressure.[3]
Reactions
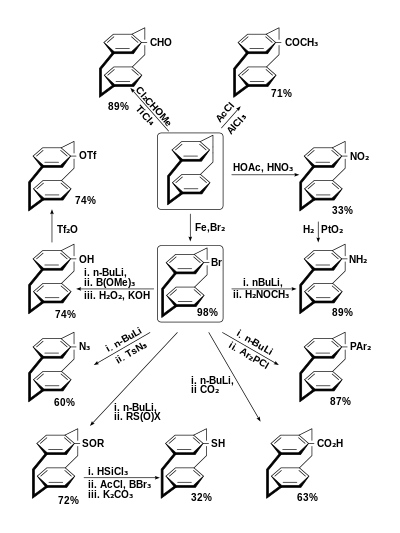
[2.2]Paracyclophane is stable under normal conditions. Its formyl, acetyl, nitro- and bromo- derivatives can be obtained by electrophilic aromatic substitution in one step.[5]
References
- ↑ Pan, Donghui; Wang, Yanbin; Xiao, Guomin (2016). "A new protocol for the synthesis of 4,7,12,15-tetrachloro[2.2paracyclophane"]. Beilstein Journal of Organic Chemistry 12: 2443–2449. doi:10.3762/bjoc.12.237. PMID 28144311.
- ↑ Wolf, Hilke; Leusser, Dirk; Jørgensen, Mads R. V.; Herbst-Irmer, Regine; Chen, Yu-Sheng; Scheidt, Ernst-Wilhelm; Scherer, Wolfgang; Iversen, Bo B. et al. (2014). "Phase Transition of [2,2]-Paracyclophane – an End to an Apparently Endless Story". Chemistry – A European Journal 20 (23): 7048–7053. doi:10.1002/chem.201304972. PMID 24740648.
- ↑ 3.0 3.1 Brown, C. J.; Farthing, A. C. (1949). "Preparation and Structure of Di-p-Xylylene". Nature 164 (4178): 915–916. doi:10.1038/164915b0. Bibcode: 1949Natur.164R.915B.
- ↑ "[2.2Paracyclophane"] (in en). https://pubchem.ncbi.nlm.nih.gov/compound/74210#section=Safety-and-Hazards.
- ↑ Hassan, Zahid; Spuling, Eduard; Knoll, Daniel M.; Bräse, Stefan (2020). "Regioselective Functionalization of [2.2Paracyclophanes: Recent Synthetic Progress and Perspectives"]. Angewandte Chemie International Edition 59 (6): 2156–2170. doi:10.1002/anie.201904863. PMID 31283092.
 |