Chemistry:(6S)-6-Fluoroshikimic acid
From HandWiki
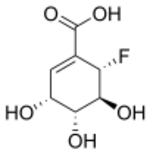
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3R,4R,5S,6S)-6-Fluoro-3,4,5-trihydroxycyclohex-1-ene-1-carboxylic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H9FO5 | |
| Molar mass | 192.14 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
(6S)-6-Fluoroshikimic acid is an antibacterial agent acting on the aromatic biosynthetic pathway.[1] It may be used against Plasmodium falciparum, the causative agent of malaria.[2] The molecule is targeting the enzymes of the shikimate pathway. This metabolic pathway is not present in mammals. The mechanism of action of the molecule is not through the inhibition of chorismate synthase but by the inhibition of 4-aminobenzoic acid synthesis.[3]
The use of the molecule led to resistances in Escherichia coli.[4]
See also
References
- ↑ "(6S)-6-Fluoroshikimic Acid, an Antibacterial Agent Acting on the Aromatic Biosynthetic Pathway". Antimicrobial Agents and Chemotherapy 38 (2): 403–406. 1994. doi:10.1128/AAC.38.2.403. PMID 8192477.
- ↑ McConkey GA (1999). "Targeting the Shikimate Pathway in the Malaria Parasite Plasmodium falciparum". Antimicrobial Agents and Chemotherapy 43 (1): 175–177. doi:10.1128/AAC.43.1.175. PMID 9869588.
- ↑ "Escherichia coli Chorismate Synthase Catalyzes the Conversion of (6S)-6-Fluoro-5-enolpyruvylshikimate-3-phosphate to 6-Fluorochorismate". Journal of Biological Chemistry 270 (39): 22811–22815. 1995. doi:10.1074/jbc.270.39.22811. PMID 7559411.
- ↑ "Frequency and Mechanism of Resistance to Antibacterial Action of ZM 240401, (6S)-6-Fluoro-Shikimic Acid". Antimicrobial Agents and Chemotherapy 39 (1): 87–93. 1995. doi:10.1128/AAC.39.1.87. PMID 7695335.
 |

