Chemistry:(Pentamethylcyclopentadienyl)titanium trichloride
From HandWiki
| |
| Names | |
|---|---|
| Other names
Cp*TiCl3
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UN number | 3261 |
| |
| |
| Properties | |
| C10H15Cl3Ti | |
| Molar mass | 289.45 g·mol−1 |
| Appearance | Orange solid |
| Melting point | 225 °C (437 °F; 498 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| P280, P305+351+338, P310 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
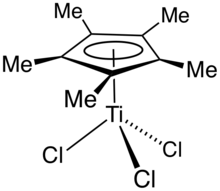
(Pentamethylcyclopentadienyl)titanium trichloride is an organotitanium compound with the formula Cp*TiCl3 (Cp* = C5(CH3)5). It is an orange solid. The compound adopts a piano stool geometry. An early synthesis involve the combination of lithium pentamethylcyclopentadienide and titanium tetrachloride.[2]
The compound is an intermediate in the synthesis of decamethyltitanocene dichloride. In the presence of organoaluminium compounds and other additives, it catalyzes the polymerization of alkenes.[3][4]
See also
References
- ↑ "Trichloro(pentamethylcyclopentadienyl)titanium(IV) 446289". https://www.sigmaaldrich.com/catalog/product/aldrich/446289.
- ↑ King, R.B.; Bisnette, M.B. (1967). "Organometallic chemistry of the transition metals XXI. Some π-pentamethylcyclopentadienyl derivatives of various transition metals". Journal of Organometallic Chemistry 8 (2): 287–297. doi:10.1016/S0022-328X(00)91042-8.
- ↑ Ishihara, N.; Kuramoto, M.; Uoi, M. (1988). "Stereospecific polymerization of styrene giving the syndiotactic polymer". Macromolecules 21 (12): 3356–3360. doi:10.1021/ma00190a003. Bibcode: 1988MaMol..21.3356I.
- ↑ Stephan, Douglas W.; Stewart, Jeffrey C.; Guérin, Frédéric; Courtenay, Silke; Kickham, James; Hollink, Emily; Beddie, Chad; Hoskin, Aaron et al. (2003). "An Approach to Catalyst Design: Cyclopentadienyl-Titanium Phosphinimide Complexes in Ethylene Polymerization". Organometallics 22 (9): 1937–1947. doi:10.1021/om020954t.
 |

