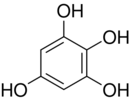
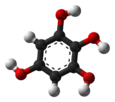
Chemistry:1,2,3,5-Tetrahydroxybenzene
From HandWiki
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Benzene-1,2,3,5-tetrol | |||
| Other names
1,2,3,5-Benzenetetrol
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C6H6O4 | |||
| Molar mass | 142.110 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
1,2,3,5-Tetrahydroxybenzene is a benzenetetrol.
It is a metabolite in the degradation of 3,4,5-trihydroxybenzoate (gallic acid) by Eubacterium oxidoreducens.[1]
The enzyme pyrogallol hydroxytransferase uses 1,2,3,5-tetrahydroxybenzene and 1,2,3-trihydroxybenzene (pyrogallol), whereas its two products are 1,3,5-trihydroxybenzene (phloroglucinol) and 1,2,3,5-tetrahydroxybenzene.[2]
Uses
1,2,3,5-Tetrahydroxybenzene, also known as pyrogallol, has various uses. It is used in the production of certain dyes, photographic developers, and hair dyes. Additionally, pyrogallol has been employed in traditional medicine and some cosmetic formulations due to its antioxidant properties.
See also
- Trihydroxybenzenes
- Pentahydroxybenzene
References
- ↑ J D Haddock, and J G Ferry (1993). "Initial steps in the anaerobic degradation of 3,4,5-trihydroxybenzoate by Eubacterium oxidoreducens: characterization of mutants and role of 1,2,3,5-tetrahydroxybenzene". J. Bacteriol. 175 (3): 669–673. doi:10.1128/jb.175.3.669-673.1993. PMID 8423143.
- ↑ Pyrogallol hydroxytransferase at www.uniprot.org
 |