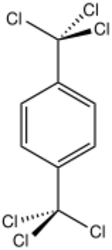
Chemistry:1,4-Bis(trichloromethyl)benzene
From HandWiki
| |
| Names | |
|---|---|
| Other names
hexachloroxylene; hexachloroparaxylene; Chloxil; Chloxyl
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Appearance | white solid |
| Density | 1.778 g/cm3 |
| Melting point | 108–110 °C (226–230 °F; 381–383 K) |
| Boiling point | 213 °C (415 °F; 486 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H301, H311, H314, H331 | |
| P260, P261, P264, P270, P271, P280, P301+310, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P311, P312, P321, P322, P330, P361, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
1,4-Bis(trichloromethyl)benzene is an organic compound with the formula C6H4(CCl3)2. A white solid, it is prepared industrially by chlorination of para-xylene. It reacts with terephthalic acid to give terephthaloyl chloride, a precursor to Kevlar.[1] It also reacts with sulfur dioxide to give the same acid chloride and thionyl chloride.[2] It reacts with hydrogen fluoride in 1,2-dichloroethane to form 1,4-bis(chlorodifluoromethyl)benzene in a yield of 79%.[3]
See also
References
- ↑ Pfoertner, Karl-Heinz (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_573.
- ↑ Rondestvedt, Christian S. (1976). "New Syntheses of Aromatic Acid Chlorides from Trichloromethylarenes. 1. Reaction with Sulfur Dioxide". The Journal of Organic Chemistry 41 (22): 3569–3574. doi:10.1021/jo00884a017.
- ↑ Dolbier, William R.; Duan, Jian-Xin; Rong, Xiao X. (2007). "Efficient synthesis of p-bis-(chlorodifluoromethyl)benzene". Journal of Fluorine Chemistry (Elsevier BV) 128 (10): 1091–1093. doi:10.1016/j.jfluchem.2007.05.007. ISSN 0022-1139.
 |

