Chemistry:19-Epivoacristine
From HandWiki
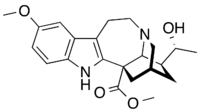
| |
| Names | |
|---|---|
| IUPAC name
Methyl (20R)-20-hydroxy-12-methoxyibogamine-18-carboxylate
| |
| Systematic IUPAC name
Methyl (6S,6aS,7S,9R)-7-[(1R)-1-hydroxyethyl]-2-methoxy-7,8,9,10,12,13-hexahydro-5H-6,9-methanopyrido[1′,2′:1,2]azepino[4,5-b]indole-6(6aH)-carboxylate | |
| Other names
20-Epivoacangarine; 19-Epi-voacangarine
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C22H28N2O4 | |
| Molar mass | 384.476 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
19-Epivoacristine is an indole alkaloid found in different species of Tabernaemontana, such as Tabernaemontana dichotoma, as well as in Peschiera affinis.[1] It is also known as 20-epivoacangarine and 19-epi-voacangarine.[2]
Potential pharmacology
19-Epivoacristine may be a selective acetylcholinesterase (AChE) inhibitor in vitro.[3]
Chemistry
19-Epivoacristine can be prepared by potassium borohydride reduction of voacryptine.[4]
See also
References
- ↑ "19-Epivoacristine, an Iboga Alkaloid Isolated from Peschiera affinis". Journal of the Brazilian Chemical Society 7 (2): 123–126. 1996. doi:10.5935/0103-5053.19960018.
- ↑ "Tabernaemontana dichotoma Roxb.ex Wall". PhytoChemical Interactions DB (PCIDB). https://www.genome.jp/db/pcidb/kna_species/4589.
- ↑ "Two fast screening methods (GC-MS and TLC-ChEI assay) for rapid evaluation of potential anticholinesterasic indole alkaloids in complex mixtures". Anais da Academia Brasileira de Ciências 80 (3): 419–26. September 2008. doi:10.1590/s0001-37652008000300003. PMID 18797794. https://www.scielo.br/pdf/aabc/v80n3/a03v80n3.pdf.
- ↑ The Alkaloids: Chemistry and Physiology. Elsevier. 12 May 2014. p. 83. ISBN 9781483221960. https://books.google.com/books?id=ovbJCgAAQBAJ&q=19-Epivoacristine&pg=PA83.
 |

