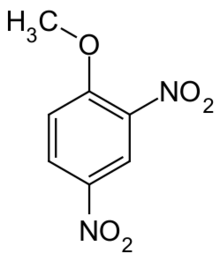
Chemistry:2,4-Dinitroanisole
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Methoxy-2,4-dinitrobenzene | |
| Other names
2,4-Dinitroanisole
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | DNAN |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H6N2O5 | |
| Molar mass | 198.134 g·mol−1 |
| Appearance | pale yellow granular crystals or needles |
| Density | 1.336 g/cm3 |
| Melting point | 94.5 °C |
| very slightly soluble | |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Warning |
| H223, H302, H351 | |
| P201, P202, P264, P270, P281, P301+312, P308+313, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,4-Dinitroanisole (DNAN) is a low sensitivity organic compound. It has an anisole (methoxybenzene) core, with two nitro groups (–NO2) attached.[1]
It is not explosive itself unless it is mixed with other explosive chemicals in certain ratios. Compared with TNT it has only 90% of the explosive power and is less dense with a higher melting point.[1]
Properties
2,4-Dinitroanisole crystallises in the monoclinic form. The unit cell has these sizes and angles: a=8.772 Å b=12.645 Å c=15.429 Å 81.89°, cell volume is V=1694 Å3, There are eight molecules in each unit cell, with four positions symmetric. The two asymmetric positions have the molecule bent in different ways. The methyl group can be rotated either at 5° or 13° out of the plane of the benzene ring. The ortho nitro group is rotated at 3° or 35°. The para nitro group is close to parallel with the ring plane.[2]
The specific heat of solid 2,4-dinitroanisole is given by Cp (Jmol−1K−1) = 0.3153 + 0.00265T (T in K). At 298.15 K. it is 219.02 Jmol−1K−1 The melting enthalpy is 20.2 kJmol−1 and solidification heat is 19.7 kJmol−1. These can differ so much as the liquid can be supercooled 22.8 °C. The initial decomposition temperature is 295 °C, and the explosion temperature is 312 °C. On explosion the adiabatic temperature rise is 4923 °C.[3] Ignition temperature is 347 °C.[1]
Reactions
2,4-Dinitroanisole reacts without heat with a potassium cyanide solution to form a red coloured product via the isopurpuric acid reaction. In this cyanide is added in the meta position, and the ortho nitro group is reduced to -NHOH.[4]
In alkaline conditions DNAN can be attacked at the methoxy position with nucleophiles to form Meisenheimer complexes. In these the ring develops a negative charge and another group is attached at the methoxy attachment point. So for example, sodium methoxide can produce the 6,6-dimethoxy-1,3-dinitro-1,3-cyclohexadiene anion.[1]
When heated under pressure with water and ammonia DNAN is converted to 2,4-dinitroaniline.[5]
With iron and acetic acid DNAN nitro groups can be reduced to amines forming 2,4-diaminoanisole.[6]
Formation
2,4-Dinitroanisole can be formed from p-nitroanisole or o-nitroanisole nitration. Also it can be formed from 1-chloro-2,4-dinitrobenzene by treatment with sodium methoxide (sodium in methanol) or sodium hydroxide with methanol.[1]
Over a period of days, alkalies will hydrolyse the ether bond to form 2,4-dinitrophenol.[7]
Use
2,4-Dinitroanisole is used as an explosive replacing TNT.[3] It is used in explosive mixtures such as IMX-101, IMX-104, PAX-48, PAX-21 and PAX-41 in the spider grenade. It can be melted and cast more safely. It has also been used as a dye ingredient and insecticide.[3]
Environment
When mixed with soil, 2,4-dinitroanisole is modified by bacteria through the path 2-nitroso-4-nitroanisole, 2-hydroxyamino-4-nitroanisole to 2-amino-4-nitroanisole. This takes place on a time scale of a few weeks. In the human body it is converted to 2,4-dinitrophenol.[8] Recent reports, demonstrated that a Nocardia sp. bacterium was able to mineralize 2, 4-dinitroanisole as a sole carbon source, via well established 2,4-dinitrophenol pathway.[9]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 Davies, Phil J.; Arthur Provatas (2006). "Characterisation of 2,4-Dinitroanisole: An Ingredient for use in Low Sensitivity Melt Cast Formulations". DSTO-TR-1904. DSTO. http://www.ntis.gov/assets/pdf/st-on-cd/ADA458880.pdf. Retrieved 12 October 2013.
- ↑ Nyburg, S. C.; Faerman, C. H.; Prasad, L.; Palleros, D.; Nudelman, N. (15 April 1987). "Structures of 2,4-dinitroanisole and 2,6-dinitroanisole". Acta Crystallographica Section C 43 (4): 686–689. doi:10.1107/S0108270187094514. Bibcode: 1987AcCrC..43..686N.
- ↑ Jump up to: 3.0 3.1 3.2 Xing, Xiaoling; Zhao, Fengqi; Ma, Shunnian; Xu, Kangzhen; Xiao, Libai; Gao, Hongxu; An, Ting; Hu, Rongzu (1 April 2012). "Specific Heat Capacity, Thermal Behavior, and Thermal Hazard of 2,4-Dinitroanisole". Propellants, Explosives, Pyrotechnics 37 (2): 179–182. doi:10.1002/prep.201000077.
- ↑ Schechter, Milton S.; Haller, H L. (1 May 1944). "Colorimetric Determination of 2,4-Dinitroanisole". Industrial & Engineering Chemistry Analytical Edition 16 (5): 325–326. doi:10.1021/i560129a017.
- ↑ Wells, F. B.; F. B. Wells; C. F. H. Allen (1935). "2,4-DINITROANILINE". Organic Syntheses 15: 22. doi:10.15227/orgsyn.015.0022. http://www.orgsyn.org/demo.aspx?prep=cv2p0221. Retrieved 13 October 2013.
- ↑ "2,4-Diaminoanisole and its Salts". IARC Monographs (International Agency for Research on Cancer) 79: 629. 2001. http://monographs.iarc.fr/ENG/Monographs/vol79/mono79-21.pdf.
- ↑ Murto, Juhani; Eero Tommila (1962). "The Influence of the Solvent on Reaction Velocity XXI The reaction of 2,4-dinitrophenyl Alkyl ethers with Hydroxyl Ion in Water and in Methanol-Water and Ethanol-Water Mixtures". Acta Chemica Scandinavica 16 (1): 63–70. doi:10.3891/acta.chem.scand.16-0063.
- ↑ Perreault, Nancy N.; Manno, Dominic; Halasz, Annamaria; Thiboutot, Sonia; Ampleman, Guy; Hawari, Jalal (1 September 2011). "Aerobic biotransformation of 2,4-dinitroanisole in soil and soil Bacillus sp". Biodegradation 23 (2): 287–295. doi:10.1007/s10532-011-9508-7. PMID 21881912. https://nrc-publications.canada.ca/eng/view/accepted/?id=126ea666-6fd7-48d1-923a-4d8cea0cd9e8.
- ↑ Fida, Tekle Tafese; Shannu Palamuru; Gunjan Pandey; Jim C. Spain (2014). "Aerobic biodegradation of 2,4-dinitroanisole (DNAN) by Nocardioides sp. JS1661". Applied and Environmental Microbiology 80 (24): 7725–31. doi:10.1128/AEM.02752-14. ISSN 0099-2240. PMID 25281383.
 |

