Chemistry:2,5-Dimethoxy-p-cymene
From HandWiki
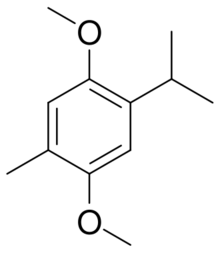
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,4-Dimethoxy-2-methyl-5-(propan-2-yl)benzene | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C12H18O2 | |
| Molar mass | 194.274 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,5-Dimethoxy-p-cymene, or thymohydroquinone dimethyl ether, is a phytochemical found in the essential oils of plants within the family Asteraceae. These essential oils, which contain the compound as a major component of the oil, have antifungal,[1] antibacterial,[2] and insecticidal[3] properties.
Natural occurrence
2,5-Dimethoxy-p-cymene occurs in a variety of different plants' essential oils. Examples include:
- Ayapana triplinervis (92.8%)[4]
- Apium leptophyllum (50.7 to 80.24%)[5]
- Cyathocline purpurea (57.4%)[2]
- Arnica montana (32.6%)[6]
- Laggera crispata (32.2%)[7]
- Blumea perrottetiana (30.0%)[3]
- Eupatorium capillifolium (20.8%)[8]
- Sphaeranthus indicus (18.2%)[9]
- Limbarda crithmoides (16.4)[10]
- Bubonium imbricatum (16.2%)[1]
Chemical synthesis
2,5-Dimethoxy-p-cymene can be synthesized from carvacrol by aromatic halogenation followed by nucleophilic substitution with sodium methoxide and Williamson ether synthesis using methyl iodide.[11]
See also
References
- ↑ 1.0 1.1 Aliou, Hakim (2008). "Chemical composition and antifungal activity of Bubonium imbricatum volatile oil". Phytopathologia Mediterranea 47 (1): 3–10. http://www.fupress.net/index.php/pm/article/view/2541. Retrieved 19 January 2017.
- ↑ 2.0 2.1 Joshi, Rajesh K. (2013). "Chemical constituents and antibacterial property of the essential oil of the roots of Cyathocline purpurea". Journal of Ethnopharmacology 145 (2): 621–625. doi:10.1016/j.jep.2012.11.045. PMID 23220198.
- ↑ 3.0 3.1 Owolabi, MS; L, Lajide; HE, Villanueva; WN, Setzer (2010). "Essential oil composition and insecticidal activity of Blumea perrottetiana growing in southwestern Nigeria". Natural Product Communications 5 (7): 1135–1138. doi:10.1177/1934578X1000500733. PMID 20734958.
- ↑ Anne Gauvin-Bialecki, Claude Marodon (November 2008). "Essential oil of Ayapana triplinervis from Reunion Island: A good natural source of thymohydroquinone dimethyl ether". Biochemical Systematics and Ecology 36 (11): 853–858. doi:10.1016/j.bse.2008.09.006.
- ↑ Pande, C.; Tewari, G.; Singh, C.; Singh, S. (2011). "Essential oil composition of aerial parts of Cyclospermum leptophyllum (Pers.) Sprague ex Britton and P. Wilson". Natural Product Research 25 (6): 592–595. doi:10.1080/14786419.2010.487190. PMID 21409720.
- ↑ Pljevljakušić, Dejan; Rančić, Dragana; Ristić, Mihailo; Vujisić, Ljubodrag; Radanović, Dragoja; Dajić-Stevanović, Zora (2012). "Rhizome and root yield of the cultivated Arnica montana L., chemical composition and histochemical localization of essential oil". Industrial Crops and Products 39: 177–189. doi:10.1016/j.indcrop.2012.02.030.
- ↑ Ram S., Verma; Rajendra C., Padalia; Chandan S., Chanotiya; Amit, Chauhan; Anju, Yadav (2011). "Chemical investigation of the essential oil of Laggera crispata (Vahl) Hepper & Wood from India". Journal of the Serbian Chemical Society 76 (4): 523–528. doi:10.2298/JSC100801048V.
- ↑ Tabanca, Nurhayat; Bernier, Ulrich R.; Tsikolia, Maia; Becnel, James J.; Sampson, Blair; Werle, Chris; Demirci, Betül; Can Ba, Kemal Hüsnü et al. (2010). "Eupatorium capillifolium Essential Oil: Chemical Composition, Antifungal Activity, and Insecticidal Activity". Natural Product Communications 5 (9): 1409–1415. doi:10.1177/1934578X1000500913. PMID 20922999. http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1975&context=usdaarsfacpub. Retrieved 19 January 2017.
- ↑ Kaul, Pran N.; Rajeswara Rao, Bhaskaruni R.; Bhattacharya, Arun K.; Singh, Kamla; Mallavarapu, Gopal R.; Ramesh, S. (2005). "Essential Oil Composition of Sphaeranthus indicus L.". Journal of Essential Oil Research 17 (4): 453–454. doi:10.1080/10412905.2005.9698961.
- ↑ Andreani, Stéphane (2013). "Chemical Variability and Antioxidant Activity of Limbarda crithmoides L. Essential Oil from Corsica". Chemistry & Biodiversity 10 (11): 2061–2077. doi:10.1002/cbdv.201300109. PMID 24243615.
- ↑ Fields, Shari L.; Soderberg, Bjora C. (1996). "Expedient Synthesis of Espintanol, p-Methoxycaracrol and Thymol Dimethyl Ether". Organic Preparations and Procedures International 28 (2): 221–225. doi:10.1080/00304949609356526.
 |

