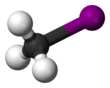
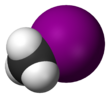
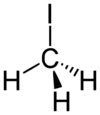
Chemistry:Methyl iodide
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Iodomethane[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations |
| ||
| 969135 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| 1233 | |||
| KEGG | |||
| MeSH | methyl+iodide | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UN number | 2644 | ||
| |||
| |||
| Properties | |||
| CH3I | |||
| Molar mass | 141.939 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | pungent, ether-like[2] | ||
| Density | 2.28 g mL−1 | ||
| Melting point | −66.5 °C; −87.6 °F; 206.7 K | ||
| Boiling point | 42.4 to 42.8 °C; 108.2 to 108.9 °F; 315.5 to 315.9 K | ||
| 14 g L−1 (at 20 °C (68 °F))[3] | |||
| log P | 1.609 | ||
| Vapor pressure | 54.4 kPa (at 20 °C (68 °F)) | ||
Henry's law
constant (kH) |
1.4 μmol Pa−1 kg−1 | ||
| -57.2·10−6 cm3/mol | |||
Refractive index (nD)
|
1.530–1.531 | ||
| Structure | |||
| Tetrahedron | |||
| Thermochemistry | |||
Heat capacity (C)
|
82.75 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−14.1–−13.1 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−808.9–−808.3 kJ mol−1 | ||
| Hazards | |||
| GHS pictograms |  
| ||
| GHS Signal word | DANGER | ||
| H301, H312, H315, H331, H335, H351 | |||
| P261, P280, P301+310, P311 | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
| ||
LC50 (median concentration)
|
1550 ppm (rat, 30 min) 860 ppm (mouse, 57 min) 220 ppm (rat, 4 hr)[4] | ||
LCLo (lowest published)
|
3800 ppm (rat, 15 min)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 5 ppm (28 mg/m3) [skin][2] | ||
REL (Recommended)
|
Ca TWA 2 ppm (10 mg/m3) [skin][2] | ||
IDLH (Immediate danger)
|
Ca [100 ppm][2] | ||
| Related compounds | |||
Related iodomethanes
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Methyl iodide, also called iodomethane, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted by rice plantations in small amounts.[5] It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups.
Preparation and handling
Methyl iodide is formed via the exothermic reaction that occurs when iodine is added to a mixture of methanol with red phosphorus.[6] The iodinating reagent is phosphorus triiodide that is formed in situ:
- 3 CH3OH + PI3 → 3 CH3I + H2PO3H
Alternatively, it is prepared from the reaction of dimethyl sulfate with potassium iodide in the presence of calcium carbonate:[6]
- (CH3O)2SO2 + KI → CH3I + CH3OSO2OK
Methyl iodide can also be prepared by the reaction of methanol with aqueous hydrogen iodide:
- CH3OH + HI → CH3I + H2O
The generated methyl iodide can be distilled from the reaction mixture.
Methyl iodide may also be prepared by treating iodoform with potassium hydroxide and dimethyl sulfate under 95% ethanol.[7]
Storage and purification
Like many organoiodide compounds, methyl iodide is typically stored in dark bottles to inhibit degradation caused by light to give iodine, giving degraded samples a purplish tinge. Commercial samples may be stabilized by copper or silver wire.[8] It can be purified by washing with Na2S2O3 to remove iodine followed by distillation.
Biogenic methyl iodide
Most methyl iodide is produced by microbial methylation of iodide. Oceans are the major source, but rice paddies are also significant.[9]
Reactions
Methylation reagent
Methyl iodide is an excellent substrate for SN2 substitution reactions. It is sterically open for attack by nucleophiles, and iodide is a good leaving group. It is used for alkylating carbon, oxygen, sulfur, nitrogen, and phosphorus nucleophiles.[8] Unfortunately, it has a high equivalent weight: one mole of methyl iodide weighs almost three times as much as one mole of methyl chloride. On the other hand, methyl chloride and methyl bromide are gaseous, thus harder to handle, and are also weaker alkylating agents.
Iodides are generally expensive relative to the more common chlorides and bromides, though methyl iodide is reasonably affordable; on a commercial scale, the more toxic dimethyl sulfate is preferred, since it is cheap and has a higher boiling point. The iodide leaving group in methyl iodide may cause unwanted side reactions. Finally, being highly reactive, methyl iodide is more dangerous for laboratory workers than related chlorides and bromides.
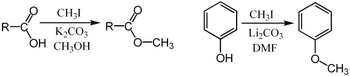
For example, it can be used for the methylation of carboxylic acids or phenols:[10]
In these examples, the base (K2CO3 or Li2CO3) removes the acidic proton to form the carboxylate or phenoxide anion, which serves as the nucleophile in the SN2 substitution.
Iodide is a "soft" anion which means that methylation with MeI tends to occur at the "softer" end of an ambidentate nucleophile. For example, reaction with thiocyanate ion favours attack at S rather than "hard" N, leading mainly to methyl thiocyanate (CH3SCN) rather than Methyl isothiocyanateCH3NCS. This behavior is relevant to the methylation of stabilized enolates such as those derived from 1,3-dicarbonyl compounds. Methylation of these and related enolates can occur on the harder oxygen atom or the (usually desired) carbon atom. With methyl iodide, C-alkylation nearly always predominates.
Other reactions
In the Monsanto process, MeI forms in situ from the reaction of methanol and hydrogen iodide. The CH3I then reacts with carbon monoxide in the presence of a rhodium complex to form acetyl iodide, the precursor to acetic acid after hydrolysis. Most acetic acid is prepared by this method.
MeI is used to prepare the Grignard reagent, methylmagnesium iodide ("MeMgI"), a common source of "Me−". The use of MeMgI has been somewhat superseded by the commercially available methyllithium. MeI can also be used to prepare dimethylmercury, by reacting 2 moles of MeI with a 2/1-molar sodium amalgam (2 moles of sodium, 1 mol of mercury).
Methyl iodide and other organic iodine compounds do form under the conditions of a serious nuclear accident,[11] after both Fukushima and Chernobyl iodine-131 in the form of organic iodine compounds were detected in Europe[12] and Japan[13] respectively.
Use as a pesticide
Methyl iodide had also been proposed for use as a fungicide, herbicide, insecticide, nematicide, and as a soil disinfectant, replacing methyl bromide (also known as bromomethane) (banned under the Montreal Protocol). Manufactured by Arysta LifeScience and sold under the brand name MIDAS, methyl iodide is registered as a pesticide in the U.S., Mexico, Morocco, Japan, Turkey, and New Zealand and registration is pending in Australia, Guatemala, Costa Rica, Chile, Egypt, Israel, South Africa and other countries.[14] The first commercial applications of MIDAS soil fumigant in California began in Fresno County, in May, 2011.[citation needed]
Methyl iodide had been approved for use as a pesticide by the United States Environmental Protection Agency in 2007 as a pre-plant biocide used to control insects, plant parasitic nematodes, soil borne pathogens, and weed seeds.[15] The compound was registered for use as a preplant soil treatment for field grown strawberries, peppers, tomatoes, grape vines, ornamentals and turf and nursery grown strawberries, stone fruits, tree nuts, and conifer trees. After the discovery phase in a consumer lawsuit, the manufacturer withdrew the fumigant citing its lack of market viability.[16]
The use of methyl iodide as a fumigant has drawn concern. For example, 54 chemists and physicians contacted the U.S. EPA in a letter, saying "We are skeptical of U.S. EPA’s conclusion that the high levels of exposure to methyl iodide that are likely to result from broadcast applications are 'acceptable' risks. U.S. EPA has made many assumptions about toxicology and exposure in the risk assessment that have not been examined by independent scientific peer reviewers for adequacy or accuracy. Additionally, none of U.S. EPA’s calculations account for the extra vulnerability of the unborn fetus and children to toxic insults."[17] EPA Assistant Administrator Jim Gulliford replied saying, "We are confident that by conducting such a rigorous analysis and developing highly restrictive provisions governing its use, there will be no risks of concern," and in October the EPA approved the use of methyl iodide as a soil fumigant in the United States.
The California Department of Pesticide Regulation (DPR) concluded that methyl iodide is "highly toxic," that "any anticipated scenario for the agricultural or structural fumigation use of this agent would result in exposures to a large number of the public and thus would have a significant adverse impact on the public health", and that adequate control of the chemical in these circumstances would be "difficult, if not impossible."[18] Methyl iodide was approved as a pesticide in California that December.[19] A lawsuit was filed on January 5, 2011, challenging California's approval of methyl iodide. Subsequently, the manufacturer withdrew the fumigant and requested that California Department of Pesticide Regulation cancel its California registration, citing its lack of market viability.[16]
Safety
Toxicity and biological effects
According to the United States Department of Agriculture methyl iodide exhibits moderate to high acute toxicity for inhalation and ingestion.[20] The Centers for Disease Control and Prevention (CDC) lists inhalation, skin absorption, ingestion, and eye contact as possible exposure routes with target organs of the eyes, skin, respiratory system, and the central nervous system. Symptoms may include eye irritation, nausea, vomiting, dizziness, ataxia, slurred speech, and dermatitis.[21] In high dose acute toxicity, as may occur in industrial accidents, toxicity includes metabolic disturbance, renal failure, venous and arterial thrombosis and encephalopathy with seizures and coma, with a characteristic pattern of brain injury.[22]
Methyl iodide has an -1">50 for oral administration to rats 76 mg/kg, and in the liver it undergoes rapid conversion to S-methylglutathione.[23]
In its risk assessment of methyl iodide, the U.S. EPA conducted an exhaustive scientific and medical literature search over the past 100 years for reported cases of human poisonings attributable to the compound. Citing the EPA as its source, the California Department of Pesticide Regulation concluded, “Over the past century, only 11 incidents of iodomethane poisoning have been reported in the published literature.” (Hermouet, C. et al. 1996 & Appel, G.B. et al. 1975) “An updated literature search on May 30, 2007 for iodomethane poisoning produced only one additional case report.” (Schwartz MD, et al. 2005). All but one were industrial—not agricultural—accidents, and the remaining case of poisoning was an apparent suicide. Methyl iodide is routinely and regularly used in industrial processes as well as in most university and college chemistry departments for study and learning related to a variety of organic chemical reactions.
Carcinogenicity in mammals
It is considered a potential occupational carcinogen by the U.S. National Institute for Occupational Safety and Health (NIOSH), the U.S. Occupational Safety and Health Administration and the U.S. Centers for Disease Control and Prevention.[24] The International Agency for Research on Cancer concluded based on studies performed after methyl iodide was Proposition 65 listed that: “Methyl iodide is not classifiable as to its carcinogenicity to humans (Group 3).” As of 2007[update] the Environmental Protection Agency classifies it as "not likely to be carcinogenic to humans in the absence of altered thyroid hormone homeostatis," i.e. it is a human carcinogen but only at doses large enough to disrupt thyroid function (via excess iodide).[25] However this finding is disputed by the Pesticide Action Network which states that the EPA’s cancer rating "appears to be based solely on a single rat inhalation study in which 66% of the control group and 54-62% of the rats in the other groups died before the end of the study". They go on to state: "The EPA appears to be dismissing early peer-reviewed studies in favor of two nonpeer-reviewed studies conducted by the registrant that are flawed in design and execution."[26] Despite requests by the U.S. EPA to the Pesticide Action Network to bring forth scientific evidence of their claims, they have not done so.
References
- ↑ 1.0 1.1 "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 657. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 2.2 2.3 NIOSH Pocket Guide to Chemical Hazards. "#0420". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0420.html.
- ↑ 3.0 3.1 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ 4.0 4.1 "Methyl iodide". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/74884.html.
- ↑ K. R. Redeker; N.-Y. Wang; J. C. Low; A. McMillan; S. C. Tyler; R. J. Cicerone (2000). "Emissions of Methyl Halides and Methane from Rice Paddies". Science 290 (5493): 966–969. doi:10.1126/science.290.5493.966. PMID 11062125. http://www.escholarship.org/uc/item/3kh8g63d.
- ↑ 6.0 6.1 King, C. S.; Hartman, W. W. (1943). "Methyl Iodide". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV2P0399.; Collective Volume, 2, pp. 399
- ↑ Kimball, R. H. (1933). "Preparation of methyl or ethyl iodide from iodoform". Journal of Chemical Education 10 (12): 747. doi:10.1021/ed010p747.
- ↑ 8.0 8.1 Sulikowski, Gary A.; Sulikowski, Michelle M.; Haukaas, Michael H.; Moon, Bongjin (2005). "Encyclopedia of Reagents for Organic Synthesis". e-EROS. doi:10.1002/047084289X.ri029m.pub2. ISBN 978-0471936237.
- ↑ Lim, Y.-K.; Phang, S.-M.; Rahman, N. Abdul; Sturges, W. T.; Malin, G. (2017). "REVIEW: Halocarbon Emissions from Marine Phytoplankton and Climate Change". Int. J. Environ. Sci. Technol.: 1355–1370. doi:10.1007/s13762-016-1219-5.
- ↑ Avila-Zárraga, J. G., Martínez, R. (January 2001). "Efficient methylation of carboxylic acids with potassium hydroxide/methyl sulfoxide and iodomethane". Synthetic Communications 31 (14): 2177–2183. doi:10.1081/SCC-100104469.
- ↑ St. John Foreman, Mark Russell (2015). "An introduction to serious nuclear accident chemistry". Cogent Chemistry 1. doi:10.1080/23312009.2015.1049111.
- ↑ Árpád Bihari, Zoltán Dezső, Tibor Bujtás, László Manga, András Lencsés, Péter Dombóvári, István Csige, Tibor Ranga, Magdolna Mogyorósi & Mihály Veres, Fission products from the damaged Fukushima reactor observed in Hungary, ISOTOPES IN ENVIRONMENTAL AND HEALTH STUDIES, 2014, volume 50, pages 94-102, DOI: 10.1080/10256016.2013.828717
- ↑ Hiroshi Noguchi and Mikio Murata, PHYSICOCHEMICAL SPECIATION OF AIRBORNE I-131 IN JAPAN FROM CHERNOBYL, 1988, JOURNAL OF ENVIRONMENTAL RADIOACTIVITY, 1988, volume 7, pages 65-74, DOI: 10.1016/0265-931X(88)90042-2
- ↑ "Iodomethane Approved in Mexico and Morocco". Business Wire. October 25, 2010. https://www.businesswire.com/news/home/20101025006234/en/Iodomethane-Approved-Mexico-Morocco. Retrieved 2018-11-25.
- ↑ Zitto, Kelly Zito, Kelly (December 2, 2010). "Methyl iodide gains state OK for use on crops". San Francisco Chronicle. http://www.sfgate.com/cgi-bin/article.cgi?f=/c/a/2010/12/01/BAOQ1GKKKN.DTL.
- ↑ 16.0 16.1 "Maker of methyl iodide scraps controversial pesticide" San Jose Mercury News March 20, 2012
- ↑ Keim, Brandon (October 1, 2007). "Scientists Stop EPA From Pushing Toxic Pesticide". Wired. https://www.wired.com/wiredscience/2007/10/scientists-stop/.
- ↑ "Report of the Scientific Review Committee on Methyl Iodide to the Department of Pesticide Regulation". special Scientific Review Committee of the California Department of Pesticide Regulation. February 5, 2010. http://www.cdpr.ca.gov/docs/risk/mei/peer_review_report.pdf.
- ↑ Schwartz, Carly (31 August 2011). "Methyl Iodide Controversy: California Officials Ignored Scientists In Approving Dangerous Pesticide". Huffington Post. https://www.huffingtonpost.com/2011/08/30/california-pesticides-officials-ignored-scientists_n_942757.html. Retrieved 2018-11-25.
- ↑ Guo, Mingxin; Gao, Suduan (2009). "Degradation of Methyl Iodide in Soil: Effects of Environmental Factors". Journal of Environmental Quality 38 (2): 513–519. doi:10.2134/jeq2008.0124. PMID 19202021. Archived from the original on August 14, 2011. https://web.archive.org/web/20110814102050/http://ddr.nal.usda.gov/bitstream/10113/28473/1/IND44184498.pdf.
- ↑ "CDC - NIOSH Pocket Guide to Chemical Hazards - Methyl iodide". cdc.gov. https://www.cdc.gov/niosh/npg/npgd0420.html. Retrieved June 25, 2016.
- ↑ Iniesta, Ivan; Radon, Mark; Pinder, Colin (2013). "Methyl iodide rhombencephalopathy: clinico-radiological features of a preventable, potentially fatal industrial accident". Practical Neurology 13 (6): 393–395. doi:10.1136/practneurol-2013-000565. http://pn.bmj.com/content/13/6/393.full.
- ↑ Johnson, M. K. (1966). "Metabolism of iodomethane in the rat". Biochem. J. 98 (1): 38–43. PMID 5938661.
- ↑ "CIB 43: MONOHALOMETHANES". Archived from the original on June 29, 2011. https://web.archive.org/web/20110629133851/http://www.cdc.gov/niosh/84117_43.html.
- ↑ "Iodomethane Pesticide Fact Sheet". 2007. http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2005-0252-0056. (36 pages, inc 12 pages of refs)
- ↑ "Archived copy". Archived from the original on July 26, 2011. https://web.archive.org/web/20110726123944/http://www.cdpr.ca.gov/docs/risk/mei/comments/panna_mei_attach1.pdf. Retrieved April 26, 2011.
Additional sources
- Sulikowski, G. A.; Sulikowski, M. M. (1999). in Coates, R.M.; Denmark, S. E. (Eds.) Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation New York: Wiley, pp. 423–26.
- Bolt H. M.; Gansewendt B. (1993). "Mechanisms of carcinogenicity of methyl halides.". Crit Rev Toxicol 23 (3): 237–53. doi:10.3109/10408449309105011. PMID 8260067.
External links
- International Chemical Safety Card 0509
- NIOSH Pocket Guide to Chemical Hazards. "#0420". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0420.html.
- IARC Summaries & Evaluations: Vol. 15 (1977), Vol. 41 (1986), Vol. 71 (1999)
- Metabolism of iodomethane in the rat
- Iodomethane NMR spectra
- Jones, Nicola (September 24, 2009). "Strawberry pesticide leaves sour taste: Methyl iodide use by Californian farmers up for review.". Nature News. doi:10.1038/news.2009.943. http://www.nature.com/news/2009/090924/full/news.2009.943.html. Retrieved September 25, 2009.
- Iodomethane in the Pesticide Properties DataBase (PPDB)