Chemistry:2,6-Dibromoquinonechlorimide
From HandWiki
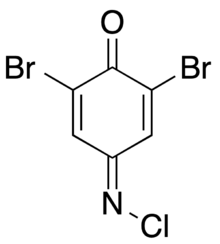
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,6-Dibromo-4-(chloroimino)cyclohexa-2,5-dien-1-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H2Br2ClNO | |
| Molar mass | 299.35 g·mol−1 |
| Appearance | yellow powder |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H240, H312, H315, H319, H335 | |
| P210, P220, P234, P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P322, P332+313, P337+313, P362, P363, P370+378, P370+380+375, P403+233, P403+235, P405, P411, P420, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,6-Dibromoquinonechlorimide is a chemical used in chemical analysis and chromatography to detect phenolic chemicals. In the presence of phenolic substances it turns indigo in colour. In the presence of aflatoxin it turns green. 2,6-Dibromoquinonechlorimide explodes if heated above 120 °C and decomposes slowly over 60 °C.[1]
2,6-Dibromoquinonechlorimide is used in a buffer solution around pH 9.4. It is very sensitive and can detect down to 0.05 parts per million of phenols. The mechanism is a reaction of the chlorimide group (=NCl) with the phenol to produce an indophenol, with two rings joined via an =N- link.[2]
References
- ↑ Lewis, Robert A. (2016) (in en). Hawley's Condensed Chemical Dictionary. John Wiley & Sons. p. 441. ISBN 978-1-118-13515-0. https://books.google.com/books?id=EwV0CgAAQBAJ&pg=PA441.
- ↑ Gibbs, H. D. (1 April 1927). "Phenol Tests. III. The Indophenol Test". The Journal of Biological Chemistry 72: 649–664. doi:10.1016/S0021-9258(18)84338-1. http://www.jbc.org/content/72/2/649.full.pdf. Retrieved 13 January 2020.
 |

