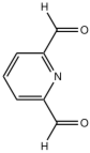
Chemistry:2,6-Diformylpyridine
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pyridine-2,6-dicarbaldehyde | |
| Other names
2,6-Pyridinedialdehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Appearance | white solid |
| Melting point | 124 °C (255 °F; 397 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2,6-Diformylpyridine is an organic compound with the formula C5H3N(CHO)2, and typically appears as a solid powder at room temperature. The molecule features formyl groups adjacent to the nitrogen of pyridine. The compound is prepared by oxidation of 2,6-dimethylpyridine.[1]
It condenses with amines to give diiminopyridine ligands,[2] as was demonstrated in Fraser Stoddart's synthesis of molecular Borromean rings.[3][4][5] It also finds use in the preparation of metal-coordinated polymer materials.[6] [7]
Related compounds
References
- ↑ Forni, Lucio; Casalone, Gianluigi (1987). "Vapour Phase Oxidation of 2,6-Lutidine to 2,6-Pyridinedicarboxaldehyde. III: Kinetic Study". Applied Catalysis 34: 317–328. doi:10.1016/S0166-9834(00)82465-3.
- ↑ Britovsek, George J. P.; Bruce, Michael; Gibson, Vernon C.; Kimberley, Brian S.; Maddox, Peter J.; Mastroianni, Sergio; McTavish, Stuart J.; Redshaw, Carl et al. (1999). "Iron and Cobalt Ethylene Polymerization Catalysts Bearing 2,6-Bis(Imino)Pyridyl Ligands: Synthesis, Structures, and Polymerization Studies". Journal of the American Chemical Society 121 (38): 8728–8740. doi:10.1021/ja990449w.
- ↑ Chichak, K. S.; Cantrill, S. J.; Pease, A. R.; Chiu, S.-H.; Cave, G. W. V.; Atwood, J. L.; Stoddart, J. F. (2004). "Molecular Borromean Rings". Science 304 (5675): 1308–1312. doi:10.1126/science.1096914. PMID 15166376. Bibcode: 2004Sci...304.1308C. http://irep.ntu.ac.uk/id/eprint/22968/1/196491_534%20Cave%20PostPrint.pdf.
- ↑ Peters, Andrea J.; Chichak, Kelly S.; Cantrill, Stuart J.; Stoddart, J. Fraser (2005). "Nanoscale Borromean links for real". Chemical Communications (27): 3394–6. doi:10.1039/B505730B. PMID 15997275.
- ↑ Yaghi, Omar M.; Kalmutzki, Markus J.; Diercks, Christian S. (2019). "Historical Perspective on the Discovery of Covalent Organic Frameworks". Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks. Wiley-VCH. p. 188. ISBN 9783527821082. https://books.google.com/books?id=06iODwAAQBAJ&pg=PA188.
- ↑ Schoustra, Sybren K.; Smulders, Maarten M. J. (2023). "Metal Coordination in Polyimine Covalent Adaptable Networks for Tunable Material Properties and Enhanced Creep Resistance". Macromolecular Rapid Communications 44 (5): 2200790. doi:10.1002/marc.202200790. PMID 36629864.
- ↑ Nasr, G.; Macron, T.; Gilles, A.; Mouline, Z.; Barboiu, M. (2012). "Metallodynameric membranes – toward the constitutional transport of gases". Chemical Communications 48 (54): 6827–6829. doi:10.1039/C2CC32656F. PMID 22652555.
