Chemistry:2-Nitrofluorene
From HandWiki
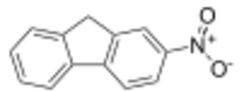
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Nitro-9H-fluorene[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| 1877983 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | 2-Nitrofluorene |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077 |
| |
| |
| Properties | |
| C13H9NO2 | |
| Molar mass | 211.220 g·mol−1 |
| Melting point | 156 to 158 °C (313 to 316 °F; 429 to 431 K) |
| log P | 3.982 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H351 | |
| P281 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2-Nitrofluorene is a by-product of combustion and is a nitrated polycyclic aromatic hydrocarbon (fluorene). 2-Nitrofluorene is listed as an IARC Group 2B carcinogen,[2] indicating it is possibly carcinogenic to humans.[3]
References
- ↑ "2-nitrofluorene - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information. Descriptors Computed from Structure. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=11831&loc=ec_rcs.
- ↑ Agents Classified by the IARC Monographs
- ↑ "2-nitrofluorene: Carcinogenic Potency Database". Berkley. http://cpdb.thomas-slone.org/chempages/2-NITROFLUORENE.html. Retrieved 7 June 2020.
 |

