Chemistry:2-tert-Butylphenol
From HandWiki
Short description: Organic aromatic compound
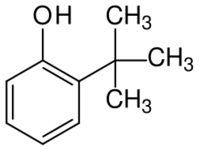
| |
| Names | |
|---|---|
| IUPAC name
2-tert-Butylphenol
| |
| Other names
o-tert-Butylphenol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3145 |
| |
| |
| Properties | |
| C10H14O | |
| Molar mass | 150.221 g·mol−1 |
| Appearance | colorless oil |
| Melting point | −7 °C (19 °F; 266 K) |
| > | |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H302, H312, H314, H332, H411 | |
| P260, P261, P264, P270, P271, P273, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P310, P312, P322, P330, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2-tert-Butyl phenol is an organic compound with the formula (CH3)3CC6H4OH. It is one of three isomeric tert-butyl phenols. It is a colorless oil that dissolves in basic water. It can be prepared by acid-catalyzed alkylation of phenol with isobutene.[2]
Uses
2-tert-Butylphenol is an intermediate in the industrial production of 2,6-di-tert-butylphenol, a common antioxidant.[2]
Hydrogenation of 2-tert-butylphenol gives cis-2-tert-butylcyclohexanol, which when acetylated is a commercial fragrance.[2]
References
- ↑ "2-Tert-butylphenol" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/6923#section=Safety-and-Hazards.
- ↑ 2.0 2.1 2.2 Fiege, Helmut; Voges, Heinz-Werner; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef et al. (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
 |

