Chemistry:5,6-Dimethylbenzimidazole
| |
| Names | |
|---|---|
| Preferred IUPAC name
5,6-Dimethyl-1H-benzimidazole | |
| Identifiers | |
3D model (JSmol)
|
|
| 116595 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| 279255 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H10N2 | |
| Molar mass | 146.193 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H315, H319, H335, H412 | |
| P261, P264, P270, P271, P273, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
5,6-Dimethylbenzimidazole is a natural benzimidazole derivative. It is a component of vitamin B12 where it serves as a ligand for the cobalt atom.[1]
5,6-Dimethylbenzimidazole is biosynthesized from flavin mononucleotide by the enzyme 5,6-dimethylbenzimidazole synthase.[2]
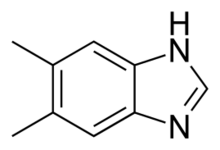
Chemical Structure
The IUPAC name for 5,6-Dimethylbenzimidazole is 1H-Benzimidazole, 5,6-dimethyl-. It consists of a benzene ring fused to an imidazole ring, with methyl groups positioned at specific carbon atoms.
Physical Properties
5,6-Dimethylbenzimidazole typically presents itself as a white to off-white crystalline solid. The compound has a molecular weight of approximately 146.19 g/mol, and its melting point falls within a specific temperature range.
Chemical Properties
The compound's chemical reactivity is attributed to the benzene and imidazole rings, enabling it to participate in various chemical reactions such as nucleophilic and electrophilic substitutions.
Occurrence and Uses
Biological Significance
Benzimidazole derivatives, including 5,6-Dimethylbenzimidazole, are known to occur in natural products and pharmaceuticals. They often exhibit diverse biological activities, making them intriguing subjects for further study.
Medicinal Chemistry
Researchers have explored the pharmacological properties of benzimidazole derivatives, with some displaying antimicrobial, antiviral, anticancer, and antifungal activities. The specific medicinal properties of 5,6-Dimethylbenzimidazole may vary based on its structural features and substituent.
Research Applications
In scientific research, 5,6-Dimethylbenzimidazole may find applications due to its unique structural characteristics. Additionally, it could serve as a valuable precursor in the synthesis of other compounds with specific desired properties.
References
- ↑ Barker, HA; Smyth, RD; Weissbach, H; Toohey, JI; Ladd, JN; Volcani, BE (1960). "Isolation and properties of crystalline cobamide coenzymes containing benzimidazole or 5, 6-dimethylbenzimidazole". The Journal of Biological Chemistry 235 (2): 480–8. doi:10.1016/S0021-9258(18)69550-X. PMID 13796809.
- ↑ "5,6-Dimethylbenzimidazole biosynthesis". MetaCyc. http://www.biocyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-5523.
 |

