Chemistry:Flavin mononucleotide
| |
| |
| Names | |
|---|---|
| IUPAC name
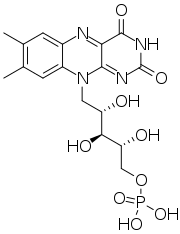
1-Deoxy-1-(7,8-dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)-D-ribitol 5-(dihydrogen phosphate)
| |
| Systematic IUPAC name
(2R,3S,4S)-5-(7,8-Dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)-2,3,4-trihydroxypentyl dihydrogen phosphate | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | Flavin+mononucleotide |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C17H21N4O9P | |
| Molar mass | 456.344 g/mol |
| Melting point | 195 °C |
| Pharmacology | |
| 1=ATC code }} | S01XA26 (WHO) |
| Ophthalmic | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Flavin mononucleotide (FMN), or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin (vitamin B2) by the enzyme riboflavin kinase and functions as the prosthetic group of various oxidoreductases, including NADH dehydrogenase, as well as a cofactor in biological blue-light photo receptors.[2] During the catalytic cycle, various oxidoreductases induce reversible interconversions between the oxidized (FMN), semiquinone (FMNH•), and reduced (FMNH2) forms of the isoalloxazine core. FMN is a stronger oxidizing agent than NAD and is particularly useful because it can take part in both one- and two-electron transfers. In its role as blue-light photo receptor, (oxidized) FMN stands out from the 'conventional' photo receptors as the signaling state and not an E/Z isomerization.
It is the principal form in which riboflavin is found in cells and tissues. It requires more energy to produce, but is more soluble than riboflavin. In cells, FMN occurs freely circulating but also in several covalently bound forms.[3] Covalently or non-covalently bound FMN is a cofactor of many enzymes playing an important pathophysiological role in cellular metabolism. For example dissociation of flavin mononucleotide from mitochondrial complex I has been shown to occur during ischemia/reperfusion brain injury during stroke.[4][5]
Food additive
Flavin mononucleotide is also used as an orange-red food colour additive, designated in Europe as E number E101a.[6]
E106, a very closely related food dye, is riboflavin-5′-phosphate sodium salt, which consists mainly of the monosodium salt of the 5′-monophosphate ester of riboflavin. It is rapidly turned to free riboflavin after ingestion. It is found in many foods for babies and young children as well as jams, milk products, and sweets and sugar products.[7]
Medical uses
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Epioxa |
| License data | |
| Identifiers | |
| DrugBank | |
Riboflavin 5'-phosphate (Epioxa) and riboflavin 5’-phosphate sodium (Epioxa HD) and are photoenhancers that are indicated for use in epithelium-on corneal collagen cross-linking for the treatment of keratoconus in people aged thirteen years of age and older, in conjunction with the O2n System and the Boost Goggles.[1]
Flavin mononucleotide, or riboflavin-5'-phosphate, is a biomolecule produced from riboflavin by the enzyme riboflavin kinase and functions as prosthetic group of various oxidoreductases including NADH dehydrogenase as well as cofactor in biological blue-light photo receptors.[8] Riboflavin 5'-phosphate sodium is a mixture of the sodium salts of riboflavin, riboflavin monophosphates, and riboflavin diphosphates.[1]
Epioxa and Epioxa HD were approved for medical use in the United States in October 2025.[9]
See also
References
- ↑ 1.0 1.1 1.2 "Prescribing information for Epioxa HD and Epioxa". https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/219910s000lbl.pdf.
- ↑ "Studies on the binding and function of flavin phosphates with flavin mononucleotide-dependent enzymes". The Journal of Biological Chemistry 241 (5): 1138–1143. March 1966. doi:10.1016/S0021-9258(18)96813-4. PMID 4379862.
- ↑ "Covalent attachment of flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) to enzymes: the current state of affairs". Protein Science 7 (1): 7–20. January 1998. doi:10.1002/pro.5560070102. PMID 9514256.
- ↑ "Critical Role of Flavin and Glutathione in Complex I-Mediated Bioenergetic Failure in Brain Ischemia/Reperfusion Injury". Stroke 49 (5): 1223–1231. May 2018. doi:10.1161/STROKEAHA.117.019687. PMID 29643256.
- ↑ "Brain Ischemia/Reperfusion Injury and Mitochondrial Complex I Damage". Biochemistry. Biokhimiia 84 (11): 1411–1423. November 2019. doi:10.1134/S0006297919110154. PMID 31760927.
- ↑ "Current EU approved additives and their E Numbers", Food Standards Agency website, retrieved 15 December 2011
- ↑ "Dietary Reference Values for riboflavin". EFSA Journal. European Food Safety Authority 15 (8): e04919. August 2017. doi:10.2903/j.efsa.2017.4919. PMID 32625611.
- ↑ "Riboflavin 5'-phosphate". 24 September 2004. https://drugs.ncats.io/substance/7N464URE7E.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Glaukos Announces FDA Approval of Epioxa" (Press release). Glaukos. 20 October 2025. Retrieved 21 October 2025.
External links
- "Flavin Mononucleotide ( Code - C61925 )". https://evsexplore.semantics.cancer.gov/evsexplore/concept/ncit/C61925.
- Clinical trial number NCT03442751 for "Study to Evaluate the Safety and Efficacy of Epi-on Corneal Cross-linking in Eyes With Progressive Keratoconus" at ClinicalTrials.gov
 |
