Chemistry:5-Hydroxyuracil
From HandWiki
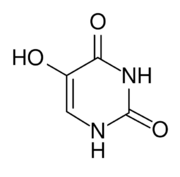
| |
| Names | |
|---|---|
| IUPAC name
2,4,5-Pyrimidinetriol
| |
| Other names
5-Hydroxy-2,4(1H,3H)-pyrimidinedione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H4N2O3 | |
| Molar mass | 128.087 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
5-Hydroxyuracil is an oxidized form of cytosine that is produced by the oxidative deamination of cytosines by reactive oxygen species.[1] It does not distort the DNA molecule and is bypassed by replicative DNA polymerases. It can miscode for adenine and is potentially mutagenic.[2]
References
- ↑ Varatharasa Thiviyanathan; Anoma Somasunderam; David E. Volka; David G. Gorenstein (2005). "5-Hydroxyuracil can form stable base pairs with all four bases in a DNA duplex". Chem. Commun. (3): 400–402. doi:10.1039/B414474K. PMID 15645051. http://pubs.rsc.org/en/Content/ArticleLanding/2005/CC/b414474k.
- ↑ Helmut Greim; Richard J. Albertini (2012). The Cellular Response to the Genotoxic Insult: The Question of Threshold for Genotoxic Carcinogens. Royal Society of Chemistry. ISBN 9781849731775. https://books.google.com/books?id=J7Kzz9kC8CkC&dq=5-hydroxycytosine&pg=PA119. Retrieved July 20, 2015.
 |
