Chemistry:6,6′-Dibromoindigo
| |
| Names | |
|---|---|
| Other names
6BrIG; Tyrian purple
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H8Br2N2O2 | |
| Molar mass | 420.060 g·mol−1 |
| Appearance | purple solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
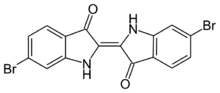
6,6'-Dibromoindigo is an organic compound with the formula (BrC6H3C(O)CNH)2. A deep purple solid, the compound is also known as Tyrian purple, a dye of historic significance. Presently, it is only a curiosity, although the related derivative indigo is of industrial significance. The molecule consists of a pair of monobrominated indole rings linked by a carbon-carbon double bond. It is produced by molluscs of the Muricidae species.[1]
The pure compound has semiconductor properties in the thin film phase, which is potentially useful for wearable electronics, and has better performance than the parent indigo in this context.[2][3]
Biosynthesis
The biosynthesis proceeds by the intermediacy of tyrindoxyl sulphate.[4]
6BrIG can also be produced enzymatically in vitro from the amino acid tryptophan. The sequence begins with bromination of the benzo ring followed by conversion to 6-bromoindole. Flavin-containing monooxygenase then couples two of these indole units to give the dye.
Chemical synthesis
The main chemical constituent of the Tyrian dye was discovered by Paul Friedländer in 1909 to be 6,6′-dibromoindigo, derivative of indigo dye, which had been synthesized in 1903.[5][6] Although the first chemical synthesis was reported in 1914, unlike indigo, it has never been synthesized at commercial level.[7][8] An efficient protocol for laboratory synthesis of dibromoindigo was developed in 2010.[9]
References
- ↑ McGovern, Patrick E.; Michel, R. H. (1990). "Royal Purple dye: The chemical reconstruction of the ancient Mediterranean industry". Accounts of Chemical Research 23 (5): 152–158. doi:10.1021/ar00173a006.
- ↑ Pandolfi, Lorenzo; Rivalta, Arianna; Salzillo, Tommaso; Giunchi, Andrea; D’Agostino, Simone; Della Valle, Raffaele G.; Brillante, Aldo; Venuti, Elisabetta (13 August 2020). "In Search of Surface-Induced Crystal Structures: The Case of Tyrian Purple". The Journal of Physical Chemistry C 124 (32): 17702–17710. doi:10.1021/acs.jpcc.0c05186.
- ↑ "Tyrian purple: The lost ancient pigment that was more valuable than gold". https://www.bbc.com/future/article/20231122-tyrian-purple-the-lost-ancient-pigment-that-was-more-valuable-than-gold.
- ↑ Valles-Regino, Roselyn; Mouatt, Peter; Rudd, David; Yee, Lachlan; Benkendorff, Kirsten (2016). "Extraction and Quantification of Bioactive Tyrian Purple Precursors: A Comparative and Validation Study from the Hypobranchial Gland of a Muricid Dicathais orbita". Molecules 21 (12): 1672. doi:10.3390/molecules21121672. PMID 27929402.
- ↑ Friedlaender, P. (1909). "Zur Kenntnis des Farbstoffes des antiken Purpurs aus Murex brandaris". Monatshefte für Chemie 30 (3): 247–253. doi:10.1007/BF01519682. http://babel.hathitrust.org/cgi/pt?id=mdp.39015031383998;view=1up;seq=257.
- ↑ Sachs, Franz; Kempf, Richard (1903). "Über p-Halogen-o-nitrobenzaldehyde". Berichte der Deutschen Chemischen Gesellschaft 36 (3): 3299–3303. doi:10.1002/cber.190303603113. https://babel.hathitrust.org/cgi/pt?id=hvd.cl1i2b;view=1up;seq=651.
- ↑ "Indigo". Encyclopædia Britannica. V (15th ed.). Chicago, IL: Encyclopædia Britannica, Inc. 1981. p. 338. ISBN 0-85229-378-X.
- ↑ Cooksey, C.J. (2001). "Tyrian purple: 6,6'-dibromoindigo and related compounds". Molecules 6 (9): 736–769. doi:10.3390/60900736. http://www.mdpi.org/molecules/papers/60900736.pdf.
- ↑ "A simple, safe and efficient synthesis of Tyrian purple (6,6'-dibromoindigo)". Molecules 15 (8): 5561–5580. August 2010. doi:10.3390/molecules15085561. PMID 20714313.
 |

