Chemistry:7α-Thiomethylspironolactone sulfoxide
From HandWiki
Short description: Chemical compound
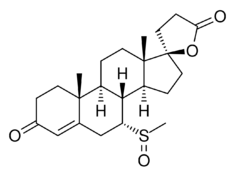 | |
| Clinical data | |
|---|---|
| Other names | 7α-TMS sulfoxide; 7α-Thiomethylspironolactone S-oxide; 7α-Methylsulfinylspironolactone |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H32O4S |
| Molar mass | 404.57 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
7α-Thiomethylspironolactone sulfoxide (also known as 7α-TMS sulfoxide, 7α-thiomethylspironolactone S-oxide, or 7α-methylsulfinylspironolactone) is a metabolite of spironolactone (brand name Aldactone), an antimineralocorticoid and antiandrogen medication.[1][2][3][4][5][6] 7α-TMS sulfoxide is specifically formed from 7α-thiomethylspironolactone (7α-TMS).[1][2][4][3][5][6]
References
- ↑ 1.0 1.1 "S-oxygenation of 7 alpha-thiomethylspironolactone by the flavin-containing monooxygenase". Drug Metabolism and Drug Interactions 6 (3–4): 337–348. 1988. doi:10.1515/dmdi.1988.6.3-4.337. PMID 3271645.
- ↑ 2.0 2.1 "Metabolism of Diuretics". Diuretics. Springer Science & Business Media. 6 December 2012. pp. 194–. ISBN 978-3-642-79565-7. https://books.google.com/books?id=zhHpCAAAQBAJ&pg=PA194.
- ↑ 3.0 3.1 Spironolactone. Analytical Profiles of Drug Substances and Excipients. 29. 2002. pp. 261–320. doi:10.1016/S1075-6280(02)29009-6. ISBN 9780122608292. https://books.google.com/books?id=RMN5zMW64ZEC&pg=PA310.
- ↑ 4.0 4.1 "Pharmacokinetics of spironolactone in man". Naunyn-Schmiedeberg's Archives of Pharmacology 296 (1): 37–45. December 1976. doi:10.1007/BF00498838. PMID 1012347.
- ↑ 5.0 5.1 "The metabolism and biopharmaceutics of spironolactone in man". Reviews on Drug Metabolism and Drug Interactions 5 (4): 273–302. 1987. doi:10.1515/DMDI.1987.5.4.273. PMID 3333882.
- ↑ 6.0 6.1 "Canrenone formation via general-base-catalyzed elimination of 7 alpha-(methylthio)spironolactone S-oxide". Chemical Research in Toxicology 2 (2): 109–113. 1989. doi:10.1021/tx00008a007. PMID 2519709.
 |

