Chemistry:Potassium canrenoate
From HandWiki
Short description: Pharmaceutical drug
 | |
| Clinical data | |
|---|---|
| Other names | SC-14266 |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intravenous |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
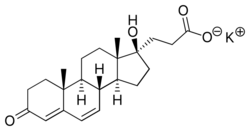
| Formula | C22H29KO4 |
| Molar mass | 396.568 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Potassium canrenoate (INN, JAN) or canrenoate potassium (USAN) (brand names Venactone, Soldactone), also known as aldadiene kalium,[1] the potassium salt of canrenoic acid, is an aldosterone antagonist of the spirolactone group.[2] Like spironolactone, it is a prodrug, and is metabolized to active canrenone in the body.[3][4]
Potassium canrenoate is notable in that it is the only clinically used antimineralocorticoid which is available for parenteral administration (specifically intravenous)[4][5] as opposed to oral administration.[6]
In the UK, it is unlicensed and only used for short term diuresis in oedema or heart failure in neonates or children under specialist initiation and monitoring.
See also
References
- ↑ Hormones and Resistance: Part 1 and. Springer Science & Business Media. 17 April 2013. pp. 186–. ISBN 978-3-642-65192-2. https://books.google.com/books?id=NdvnCAAAQBAJ&pg=PA186.
- ↑ Dictionary of Steroids. CRC Press. 23 May 1991. pp. 656–. ISBN 978-0-412-27060-4. https://books.google.com/books?id=qw5X0NK1A90C&pg=PA656.
- ↑ Burger's Medicinal Chemistry and Drug Discovery: Therapeutic agents. Wiley. 1996. ISBN 978-0-471-57557-3. https://books.google.com/books?id=iLvwAAAAMAAJ.
- ↑ 4.0 4.1 "Diurectics and the Critical Ill". Oxford Desk Reference: Critical Care. OUP Oxford. 27 November 2008. pp. 187–. ISBN 978-0-19-922958-1. https://books.google.com/books?id=eLqMpXfAlEcC&pg=PA187.
- ↑ "Non-resection: Radiofrequency Ablation, Cryo, Microwave". Surgical Principles of Minimally Invasive Procedures: Manual of the European Association of Endoscopic Surgery (EAES). Springer. 21 June 2017. pp. 136–. ISBN 978-3-319-43196-3. https://books.google.com/books?id=tAImDwAAQBAJ&pg=PA136.
- ↑ "30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid receptor antagonists: 60 years of research and development". The Journal of Endocrinology 234 (1): T125–T140. July 2017. doi:10.1530/JOE-16-0600. PMID 28634268.
{{Navbox
| name = Androgens and antiandrogens | title = Androgens and antiandrogens | state = collapsed | listclass = hlist | groupstyle = text-align:center;
| group1 = Androgens
(incl. AAS)
| list1 =
| group2 = Antiandrogens | list2 = {{Navbox|child | groupstyle = text-align:center; | groupwidth = 9em;
| group1 = AR antagonists | list1 =
- Steroidal: Abiraterone acetate
- Canrenone
- Chlormadinone acetate
- Cyproterone acetate
- Delmadinone acetate
- Dienogest
- Drospirenone
- Medrogestone
- Megestrol acetate
- Nomegestrol acetate
- Osaterone acetate
- Oxendolone
- Potassium canrenoate
- Spironolactone
- Nonsteroidal: Apalutamide
- Bicalutamide
- Cimetidine
- Darolutamide
- Enzalutamide
- Flutamide
- Ketoconazole
- Nilutamide
- Seviteronel†
- Topilutamide (fluridil)
| group2 = Steroidogenesis| list2 =
inhibitors
| 5α-Reductase | |
|---|---|
| Others |
| group3 = Antigonadotropins | list3 =
- D2 receptor antagonists (prolactin releasers) (e.g., domperidone, metoclopramide, risperidone, haloperidol, chlorpromazine, sulpiride)
- Estrogens (e.g., bifluranol, [[diethylstilbestrol, estradiol, estradiol esters, ethinylestradiol, ethinylestradiol sulfonate, paroxypropione)
- GnRH agonists (e.g., leuprorelin)
- GnRH antagonists (e.g., cetrorelix)
- Progestogens (incl., chlormadinone acetate, [[cyproterone acetate, hydroxyprogesterone caproate, gestonorone caproate, [[Chemistry:Medroxyprogesterone medroxyprogesterone acetate, Chemistry:Megestrol acetate|megestrol acetate]])
| group4 = Others | list4 =
- Androstenedione immunogens: Androvax (androstenedione albumin)
- Ovandrotone albumin (Fecundin)
}}
| liststyle = background:#DDDDFF;| list3 =
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
- See also
- Androgen receptor modulators
- Estrogens and antiestrogens
- Progestogens and antiprogestogens
- List of androgens/anabolic steroids
}}
 |

