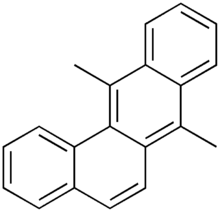
Chemistry:7,12-Dimethylbenz(a)anthracene
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
7,12-Dimethyltetraphene | |
| Other names
7,12-Dimethylbenzo[a]phenanthrene
7,12-Dimethylbenzanthracene 7,12-Dimethyltetraphene 1,4-Dimethyl-2,3-benzophenanthrene | |
| Identifiers | |
3D model (JSmol)
|
|
| 1912135 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| 263937 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077 |
| |
| |
| Properties | |
| C20H16 | |
| Molar mass | 256.348 g·mol−1 |
| Melting point | 122 to 123 °C (252 to 253 °F; 395 to 396 K) |
| Hazards | |
| Main hazards | T (Toxic) |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H350 | |
| P201, P202, P264, P270, P281, P301+312, P308+313, P330, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
7,12-Dimethylbenz[a]anthracene (DMBA) is an immunosuppressor and a powerful organ-specific laboratory carcinogen.[2] DMBA is widely used in many research laboratories studying cancer. DMBA serves as a tumor initiator. Tumor promotion can be induced with treatments of 12-O-tetradecanoylphorbol-13-acetate (TPA) in some models of two-stage carcinogenesis.[3] This allows for a greatly accelerated rate of tumor growth, making many cancer studies possible.
References
- ↑ 7,12-Dimethylbenz(a)anthracene at Sigma-Aldrich
- ↑ Miyata M; Furukawa M; Takahashi K; Gonzalez FJ; Yamazoe Y (2001). "Mechanism of 7, 12-Dimethylbenz[a]anthracene-Induced Immunotoxicity: Role of Metabolic Activation at the Target Organ". Jpn J Pharmacol 86 (3): 302–309. doi:10.1254/jjp.86.302. PMID 11488430.
- ↑ Sung YM; He G; Fischer, SM (2005). "Lack of Expression of the EP2 but not EP3 Receptor for Prostaglandin E2 Results in Suppression of Skin Tumor Development". Cancer Res 65 (20): 9304–9311. doi:10.1158/0008-5472.can-05-1015. PMID 16230392.
 |


