Chemistry:9-Crown-3
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,4,7-Trioxonane | |
| Other names
1,4,7-Trioxacyclononane; Ethylene oxide trimer
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1421638 | |
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C6H12O3 | |
| Molar mass | 132.159 g·mol−1 |
| Appearance | colorless liquid |
| Melting point | 0 °C (32 °F; 273 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
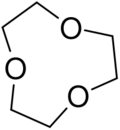
9-Crown-3, also called 1,4,7-trioxonane or 1,4,7-trioxacyclononane is a crown ether with the formula (C2H4O)3. A colorless liquid, it is obtained in low yield by the acid-catalyzed oligomerization of ethylene oxide.[1]
In contrast to larger crown ethers (12-crown-4, and 18-crown-6), 9-crown-3 has elicited very little interest, except from theorists.[2][3]
See also
References
- ↑ Dale, Johannes; Borgen, Gerd; Daasvatn, Kari; Liaaen-Jensen, Synnøve; Enzell, Curt R.; Mannervik, Bengt (1974). "The Oligomerization of Ethylene Oxide to Macrocyclic Ethers, Including 1,4,7-Trioxacyclononane". Acta Chemica Scandinavica 28b: 378–379. doi:10.3891/acta.chem.scand.28b-0378.
- ↑ Anderson, Wayne P.; Behm, Philip; Glennon, Timothy M.; Zerner, Michael C. (1997-03-01). "Quantum Mechanics and Molecular Mechanics Studies of the Low-Energy Conformations of 9-Crown-3". The Journal of Physical Chemistry A 101 (10): 1920–1926. doi:10.1021/jp962172h. ISSN 1089-5639. Bibcode: 1997JPCA..101.1920A. http://dx.doi.org/10.1021/jp962172h.
- ↑ Jagannadh, B.; Sarma, Jagarlapudi A. R. P. (1999-12-01). "Searching the Conformational Space of Cyclic Molecules: A Molecular Mechanics and Density Functional Theory Study of 9-Crown-3" (in en). The Journal of Physical Chemistry A 103 (50): 10993–10997. doi:10.1021/jp991201w. ISSN 1089-5639. Bibcode: 1999JPCA..10310993J. https://pubs.acs.org/doi/10.1021/jp991201w.
 |

