Chemistry:Adenosine 5'-tetraphosphate
From HandWiki
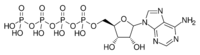
| |
| Names | |
|---|---|
| IUPAC name
Adenosine 5′-(pentahydrogen tetraphosphate)
| |
| Systematic IUPAC name
O1-{[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl} pentahydrogen tetraphosphate | |
| Other names
Adenosine tetraphosphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H17N5O16P4 | |
| Molar mass | 587.160 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Adenosine 5′-tetraphosphate, Ap4 or ATPP is a nucleotide. It is produced from ATP and triphosphate (P3) through the action of acetyl—CoA synthetase.[1] Acetyl—CoA synthetase also produces adenosine 5'-pentaphosphate through the reaction of ADP and tetraphosphate (P4).
Functions
ATPP has been found to play physiological roles in some mammals.
Rabbits
ATPP is a constituent of aqueous humor in rabbits, where it was found to reduce the intraocular pressure.[2]
Rats
ATPP has been suggested to play a regulatory role in rat aorta.
References
- ↑ Guranowski, A.; Günther Sillero, M.A.; Sillero, A. (1994). "Adenosine 5′-tetraphosphate and adenosine 5′-pentaphosphate are synthesized by yeast acetyl coenzyme A synthetase.". J Bacteriol 176 (10): 2986–90. doi:10.1128/jb.176.10.2986-2990.1994. PMID 7910605.
- ↑ Pintor, Jesús; Peláez, Teresa; Peral, Assumpta (February 2004). "Adenosine tetraphosphate, Ap4, a physiological regulator of intraocular pressure in normotensive rabbit eyes". The Journal of Pharmacology and Experimental Therapeutics 308 (2): 468–473. doi:10.1124/jpet.103.058669. ISSN 0022-3565. PMID 14600249.
 |
