Chemistry:Adipamide
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexanediamide | |
| Other names
Hexanedioic diamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| 4-02-00-01972 | |
| ChemSpider | |
| EC Number |
|
| MeSH | Adipamide |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C6H12N2O2 | |
| Molar mass | 144.174 g·mol−1 |
| Appearance | powder |
| Melting point | 220 to 225 °C (428 to 437 °F; 493 to 498 K) |
| 4.4 g/L (12 °C) | |
| Related compounds | |
Related compounds
|
hexanedioic acid hexanedihydrazide hexanedioyl dichloride hexanedinitrile |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
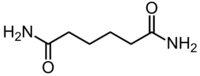
Adipamide is the organic compound with the formula (CH2CH2C(O)NH2)2. It is a white solid. The dominant commercial interest in adipamides is related to their presence in nylons.
Adipamide is formed by treating dimethyl adipate with concentrated ammonia.[1][2]
References
- ↑ Musser, M. T. (2005). "Adipic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_269. ISBN 3527306730.
- ↑ "Dimethyl Adipate". chemicalland21.com. http://www.chemicalland21.com/industrialchem/solalc/DIMETHYL%20ADIPATE.htm.
External links
 |
